4 mg powder and liquid for injection, solution
somatropin
What Zomacton is and what it is used for
Zomacton contains the active substance somatropin, also known as growth hormone.
Growth hormone is produced naturally in the body and has an important role in growth.
Zomacton contains somatropin manufactured in a pharmaceutical manufacturing plant.
Zomacton is used for long-term treatment of:
- Children who have a growth disorder due to insufficient production of growth hormone.
- Short stature due to Turner syndrome (a genetic disorder that can affect women).
The somatropin contained in Zomacton may also be approved to treat other conditions not mentioned in this leaflet. Ask your doctor, pharmacist, or another healthcare professional if you have any further questions, and always follow their instructions.
What you need to know before you use Zomacton
Do not use Zomacton
- if you are allergic to somatropin or any of the other ingredients of this medicine (listed in section 6)
- for children with closed epiphyses (children who have stopped growing)
- do not use Zomacton and tell your doctor if you have an active tumor (cancer); tumors must be inactive and you must have finished your tumor treatment before starting treatment with Zomacton
- to premature or newborn babies because the diluent contains benzyl alcohol
- in case of acute critical medical conditions such as complications for example after open heart or abdominal surgery, multiple trauma from an accident, or breathing problems
- to children with chronic kidney disease during kidney transplantation
Warnings and precautions
Talk to your doctor before using Zomacton.
Treatment with Zomacton should only take place under the supervision of a qualified physician experienced in the treatment of patients with growth hormone deficiency.
- As benzyl alcohol is included as an excipient, Zomacton can cause poisoning and allergic reactions in children under 3 years of age and should not be given to premature babies or newborns.
- Patients with Prader-Willi syndrome should not be treated with Zomacton unless they also suffer from growth hormone deficiency.
- If you have a family history of diabetes, your blood sugar levels may need to be checked from time to time by your doctor.
- If you have diabetes, your blood glucose levels need to be checked regularly and your dose may need to be adjusted to maintain diabetic control. Your doctor will let you know if this is necessary.
- If your growth hormone deficiency is due to an intracranial injury, you should be closely monitored for worsening or recurrence of the injury. If this happens, your doctor will tell you if you need to stop taking Zomacton.
- Tell your doctor if you notice signs or symptoms of recurrence of previous malignancies.
- If you are on glucocorticoid replacement therapy, you should regularly contact your doctor, as you may need to adjust your glucocorticoid dose.
- If you develop signs or symptoms of the following while you are being treated with Zomacton, contact your doctor or emergency department immediately:
- repeated or severe headaches
- vision problems
- nausea and/or vomiting
- Disturbances in thyroid function may occur during treatment with Zomacton. This may require a change in therapy. Your doctor will usually ask you to have regular tests to make sure your thyroid is working properly.
- Some children with growth hormone deficiency have developed leukemia (increased amount of white blood cells ) regardless of whether they have received growth hormone treatment or not. However, there is no evidence that the frequency of leukemia would be greater than in the general population. No link between growth hormones and leukemia has been proven.
- Consult your doctor if you develop lameness or pain in your hip or knee.
- If you suffer from complications after surgery, trauma, or acute breathing problems.
- If you need surgery, are seriously injured in an accident, or become seriously ill, your doctor may need to review your treatment.
Other medicines and Zomacton
Tell your doctor or pharmacist if you are using, have recently used, or might be using other medicines.
- Patients treated with glucocorticoid er may need to have their dose carefully adjusted because glucocorticoid er may inhibit the growth-promoting effect of somatropin. Therefore, tell your doctor if you are being treated with steroids for insufficient production of ACTH (adrenocorticotropic hormone ).
- Androgens, estrogens, and anabolic steroids can accelerate bone maturation and thus reduce the overall growth rate.
- As somatropin can cause insulin resistance, the insulin dose may need to be adjusted in patients with diabetes.
- Tell your doctor if you are regularly treated with prescription drugs such as steroids, anti-epileptic drugs, or drugs that weaken the body’s immune system.
Pregnancy and breastfeeding
There is no experience of use in pregnant women. Zomacton should therefore not be used during pregnancy.
It is unknown whether Zomacton passes into breast milk. Zomacton should therefore not be used during breastfeeding.
Driving ability and use of machinery
Zomacton has no or negligible effect on the ability to drive or use machines.
You are always responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. A description of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. Discuss with your doctor or pharmacist if you are unsure.
Zomacton contains benzyl alcohol 9 mg/ml
As benzyl alcohol is included as an excipient, Zomacton can cause poisoning and allergic reactions in children under 3 years of age and should not be given to premature babies or newborns.
How to use Zomacton
Always use Zomacton as directed by your doctor. Ask your doctor or pharmacist if you are unsure.
Your doctor or nurse will usually decide with you which is the most appropriate method of administration and provide you with dosing instructions for the method used. Dose one is administered subcutaneously (under the skin) with a syringe or with a needle-free injection aid, Zomajet 4, or with Ferring-Pen, an injection aid with a needle.
Dosage:
Growth hormone deficiency in children:
Your doctor will calculate the exact dose for you based on your body weight. Generally, a dose of 0.17-0.23 mg per kg of body weight per week is recommended. The weekly dose can be divided into six or seven doses, corresponding to a daily dose of 0.02-0.03 mg per kg of body weight. The maximum recommended dose per week is 0.27 mg per kg of body weight, corresponding to a daily dose of 0.04 mg per kg of body weight.
Turner syndrome (females only):
Your doctor will calculate the exact dose for you based on your body weight. Generally, a dose of 0.33 mg per kg of body weight per week is recommended. The weekly dose can be divided into six or seven doses, corresponding to a daily dose of 0.05 mg per kg of body weight.
Instructions for use for the preparation of solution
The powder should only be dissolved in the supplied diluent
Two strengths can be prepared depending on the volume of solvent used. The doctor will tell you what strength to use.
- for use of the Zomajet 4, Ferring-Pen, or a regular syringe, use 1.3 ml of the solvent and prepare a solution that is 3.3 mg/ml
- for use of a regular syringe, use 3.2 ml of the solvent and prepare a solution that is 1.3 mg/ml
Preparation must take place by good aseptic practice.
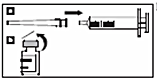
1a. Attach the needle to the graduated syringe.
1b. Open the plastic cap on the vial.
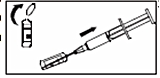
2. Break off the top of the ampoule one. Remove the plastic cover from the needle. Make sure the plunger is fully depressed before inserting the needle into ampoule one. Slowly draw up the required volume into the syringe.
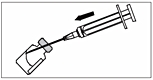
3. To avoid foaming, inject the solvent along the wall of the vial.
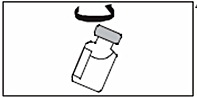
4. Swirl the vial gently to mix the solution into a clear, colorless solution. As the powder mainly contains protein , the solution should not be shaken.
If the solution is cloudy or contains particles, the vial and its contents should be discarded.
If the solution is cloudy after refrigerated storage, allow it to reach room temperature (25°C). If the turbidity still persists or if the solution is colored, discard the vial and its contents.
If the solution is stored in a refrigerator, it can be used for up to 14 days after preparation . Unused solution must be discarded after 14 days of storage.
Administration
The clear, colorless solution should be injected subcutaneously as shown either by syringe or Zomajet 4 or Ferring Pen.
ZomaJet 4 (an injection device without a needle) and Ferring-Pen (an injection device with a needle) are not provided in the package)
Below is a general description of the use of the adapter prior to the administration procedure with Zomajet 4. Administration should be performed using good aseptic practice .
- Wash hands thoroughly.
- Wipe the top of the vial with antiseptic solution to avoid contamination of the contents.
- Center the tip of the adapter on the vial membrane and push the adapter down until it attaches to the vial. Twist the adapter gently when connecting to the vial to allow the adapter tip to easily puncture the vial membrane .
- To remove the adapter cap, hold the vial and adapter with one hand and pull the cap straight up. Save the lid for later storage.
The steps for positioning the adapter for use with the ZomaJet 4 are shown below. Instructions for using the ZomaJet 4 can be found in a leaflet enclosed with the device.
If you use too much Zomacton
An overdose can lead to hypoglycemia (low blood sugar), followed by hyperglycemia (high blood sugar).
If you have ingested too much medicine or if, for example, a child has accidentally ingested the medicine, always contact a doctor or hospital for an assessment of the risk and advice.
The effect of repeated overdose is unknown.
If you forget to take Zomacton
Do not worry if you missed a dose . Continue as usual and take the next dose at the usual time. You may suffer from hypoglycaemia (low blood sugar level). Even if the long-term effect of the treatment is not affected, contact your doctor if this occurs.
Possible side effects
Like all medicines, this medicine can cause side effects , although not everybody gets them.
Administration of growth hormone by subcutaneous injection may cause a decrease or increase in the amount of adipose tissue at the injection site. It is therefore recommended to frequently vary the injection site. In rare cases, patients may develop pain and an itchy rash at the injection site.
Very common (may affect more than 1 in 10 users)
Adults only:
- swelling due to fluid accumulation, especially in the hands and feet ( edema )
- slightly elevated blood sugar ( hyperglycemia )
- joint pain ( arthralgia )
- muscle pain ( myalgia )
- headache
- tingling ( paresthesia )
Common (may affect up to 1 in 10 users)
Children and adults:
- reduced function of the thyroid gland
- immune reaction against growth hormone et which can be shown with a blood test (antibody formation)
- headache
- increased muscle tension
Children only:
- swelling due to fluid accumulation, especially in the hands and feet ( oedema , peripheral oedema )
- injection site reactions
- weakness ( asthenia )
- impaired glucose tolerance
- joint pain ( arthralgia )
- muscle pain ( myalgia )
Adults only:
- stiffness in arms and/or legs
- difficulty falling asleep and/or difficulty staying asleep (insomnia)
Uncommon (may affect up to 1 in 100 users)
Children and adults:
- anemia
- fast heartbeat ( tachycardia )
- feeling of rotation (vertigo)
- double vision ( diplopia )
- papilledema
- vomiting, abdominal pain, flatulence, nausea
- weakness
- bruising, bleeding, swelling or tissue enlargement at the injection site
- low blood sugar ( hypoglycemia )
- increased phosphate content in the blood
- muscle wasting
- bone pain
- carpal tunnel syndrome
- tumors, even malignant
- sleepiness ( somnolence )
- involuntary eye movements ( nystagmus )
- personality changes
- urinary tract problems such as urinary incontinence, blood in the urine, increased urine volume, need to urinate more often
- injection site reactions (including decreased amount of adipose tissue, skin thinning, scaling, hives, increased hair, skin enlargement)
Children only:
- stiffness in arms and legs
Adults only:
- high blood pressure ( hypertension )
Rare (may affect up to 1 in 1,000 users)
Children and adults:
- diarrhea
- abnormal kidney function test
- diabetes mellitus type II
- tingling and numbness in certain parts of the body ( neuropathy )
- fluid accumulation in the brain (appears as recurrent or severe headaches, blurred vision and nausea and/or vomiting)
Children only:
- high blood pressure ( hypertension )
- difficulty falling asleep and/or difficulty staying asleep (insomnia)
- numbness, burning, a burning sensation or tingling ( paresthesia )
Very rare (may affect up to 1 in 10,000 users)
Children only:
- Leukemia (does not appear to be more common than in children in the general population)
- Abnormal breast enlargement ( gynecomastia )
How to store Zomacton
Keep this medicine out of the sight and reach of children.
Use Zomacton before the expiry date which is stated on the carton. The expiration date is the last day of the specified month.
Store in a refrigerator, 2°C-8°C. Store in the outer carton. Light sensitive.
When the powder has been dissolved with the supplied diluent , store the vial upright in a refrigerator at 2°C-8°C.
After preparation , the solution must be used within 14 days. You should discard any solution remaining in the vial at the end of this period.
If the solution is cloudy after refrigerated storage, allow it to reach room temperature (25°C). If turbidity persists or discoloration occurs, discard the vial and its contents.
Medicines must not be thrown into the drain or among household waste. Ask the pharmacist how to dispose of medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
- Each vial of powder contains the active substance somatropin 4 mg (1.3 mg/ml or 3.3 mg/ml after reconstitution )
- Other ingredients are:
Powder: Mannitol
Solvent: benzyl alcohol 9 mg/ml, sodium chloride and water for injections
Zomacton contains less than 1 mmol sodium chloride (23 mg) per dose , i.e. it is almost “sodium-free”.
Appearance and package sizes of the medicine
The product is powder and liquid for injection , solution.
The powder is white to off-white in color. When dissolved in the supplied liquid, a clear, colorless solution is formed.
The powder is supplied in a vial (4 mg somatropin) and the liquid in an ampoule (3.5 ml) in three different packaging types.
- Pack sizes: 1, 5 or 10
or
- With syringe and needle
Package size: 5
or
- With syringe , needle and adapter
Pack size 1, 5 or 10, for use with needle-free injection aid or injection aid with needle (not provided in the package)
Not all pack sizes may be marketed.
Marketing Authorisation Holder:
Ferring Läkemedel AB
Box 4041
203 11 Malmö
040-691 69 00
Manufacturer:
Ferring GmbH
Wittland 11, D-24109 Kiel
Germany
This medicine is approved within the European Economic Area under the names:
Austria, Belgium, Denmark, Finland, France, Germany, Greece, Ireland, Italy, Luxembourg, Netherlands, Portugal, Spain, United Kingdom: Zomacton