What Zavesca is and what it is used for
Zavesca contains the active substance miglustat which belongs to a group of medicines that affect metabolism. It is used to treat two diseases:
- Zavesca is used to treat mild to moderate type 1 Gaucher disease in adults.
For type 1 Gaucher disease, a substance called glucosylceramide is not removed from your body. It begins to be stored in certain cells that belong to the body’s immune system. This can lead to liver and spleen enlargement, changes in the blood, and bone disease.
Gaucher disease type 1 is normally treated with enzyme replacement therapy. Zavesca is only used if the patient is not considered suitable for treatment with enzyme replacement therapy.
- Zavesca is also used to treat worsening neurological symptoms in Niemann-Pick disease type C in adults and children.
If you have Niemann-Pick disease type C, fats, such as glycosphingolipids, are stored in your brain cells. This can lead to disturbances in neurological functions such as slow eye movements, balance, swallowing ability, memory, and to convulsions.
Zavesca works by inhibiting an enzyme called ‘glucosylceramide synthetase’, which is responsible for the first step in the production of most glycosphingolipids.
What you need to know before you take Zavesca
Do not take Zavesca:
- if you are allergic to miglustat or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before taking Zavesca
- if you have any kidney disease
- if you have any liver disease
Your doctor will carry out the following checks before and during treatment with Zavesca:
- examination to check the nerves in your arms and legs
- measurement of vitamin B 12 value
- follow your growth if you are a child or adolescent with Niemann-Pick type C disease
- check the platelet count
The reason for these checks is that some patients have experienced tingling or numbness in the hands and feet or lost weight during treatment with Zavesca. The checks make it easier for the doctor to decide whether these problems are due to your illness or something you have had in the past, or whether they are due to side effects of Zavesca (for more information see section 4).
If you get diarrhea, your doctor may ask you to change your diet so that you reduce your intake of milk sugar and carbohydrates such as sucrose (plain sugar). The doctor may also ask you not to take Zavesca with food. In some cases, the doctor may temporarily lower your dose or prescribe an anti-diarrhea medicine (such as loperamide). If your diarrhea does not respond to these measures, or if you have any other stomach problems, consult your doctor. In such a case, your doctor may decide on further investigations.
Male patients should use reliable contraception during treatment with Zavesca and for 3 months after stopping treatment.
Children and young people
Do not give this medicine to children and adolescents (under 18 years of age) with type 1 Gaucher disease because it is not known whether it works against that disease.
Other medicines and Zavesca
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Tell your doctor if you are taking medicines containing imiglucerase, which is sometimes used at the same time as Zavesca. They can lower the amount of Zavesca in your body.
Pregnancy, breastfeeding, and fertility
You should not take Zavesca if you are pregnant or planning to become pregnant. Your doctor can give you more information. You must use safe contraception while taking Zavesca. Do not breastfeed while taking Zavesca.
Male patients should use reliable contraceptive methods during treatment with Zavesca and for 3 months after stopping treatment.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Driving ability and use of machinery
Zavesca may make you feel dizzy. Do not drive a vehicle or use any tools or machinery if you feel dizzy.
Zavesca contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per capsule, i.e. it is almost “sodium-free”.
How to take Zavesca
Always take this medicine as directed by your doctor. Ask your doctor or pharmacist if you are unsure.
- For Gaucher disease type 1: for adult patients, the usual dose is one capsule (100 mg) three times a day (morning, afternoon, and evening). This means a maximum daily dose of three capsules (300 mg).
- For Niemann-Pick disease type C: For adults and adolescents (over 12 years of age), the usual dose is one or two capsules (200 mg) three times a day (morning, afternoon, and evening). This means a maximum daily dose of 6 capsules (600 mg).
For children under the age of 12 who have Niemann-Pick disease type C, the doctor will adjust the dose one.
If you have problems with your kidneys, you may receive a lower starting dose. Your doctor can lower your dose, e.g. to one capsule (100 mg) once or twice a day if you suffer from diarrhea during treatment with Zavesca (see section 4). Your doctor will tell you how long you will be treated.
Take out the capsule as follows :
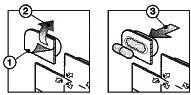
- Separate at the perforations
- Pull the paper back at the arrows
- Push the medicine through the foil
Zavesca can be taken with or without food. You should swallow the capsule whole with a glass of water.
If you have taken too much Zavesca
Consult your doctor immediately if you have taken more capsules than you should. Zavesca has been used in clinical trials at doses up to 3,000 mg: this caused a decrease in the number of white blood cells and other side effects similar to those described in section 4.
If you forget to take Zavesca
Take the next capsule at the usual time. Do not take a double dose to make up for a missed dose.
If you stop taking Zavesca
Do not stop taking Zavesca without talking to your doctor.
If you have further questions about this medicine, contact your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Most serious side effects:
Some patients have experienced tingling or numbness in the hands and feet (common). This may be a sign of peripheral neuropathy due to side effects of Zavesca or due to pre-existing conditions. Your doctor will carry out some checks before and during treatment with Zavesca to assess this (see section 2).
If you experience any of these symptoms, consult your doctor as soon as possible.
If you get mild tremors, usually in the hands, consult your doctor as soon as possible. These tremors usually disappear without having to stop the treatment. Sometimes your doctor may need to reduce your dose or stop Zavesca to help you get rid of your tremors.
Very common: (may affect more than 1 in 10 people)
The most common side effects are diarrhea, gas, abdominal (stomach) pain, weight loss, and decreased appetite.
Do not worry if you lose some weight when you start treatment with Zavesca. Usually, you stop losing weight as the treatment continues.
Common: (may affect up to 1 in 10 people)
Common side effects of treatment include headache, dizziness, paraesthesia (tingling or numbness), abnormal coordination, hypoesthesia (diminished sensation), acid reflux (heartburn), nausea, constipation and vomiting, abdominal (stomach) swelling or discomfort, and thrombocytopenia (decreased level of platelets ). The neurological symptoms and thrombocytopenia may be caused by the underlying disease.
Other possible side effects are muscle spasms or weakness, fatigue, chills and general malaise, depression, difficulty sleeping, forgetfulness, and reduced libido.
Most patients experience one or more of these side effects, usually at the beginning of treatment or periodically during treatment. In most cases, side effects are mild and disappear fairly quickly. If any of these side effects cause problems, consult your doctor. He or she can then reduce the dose of Zavesca or suggest other medications that can help reduce the side effects.
How to store Zavesca
Keep this medicine out of the sight and reach of children.
Use before the expiry date stated on the carton after “EXP”. The expiration date is the last day of the specified month.
Store at a maximum of 30 °C.
Medicines must not be thrown into the drain or among the household waste. Ask the pharmacist how to dispose of medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
The active substance is miglustat 100 mg.
Other ingredients are:
Sodium starch glycolate,
Povidone (K30),
Magnesium stearate.
Gelatine,
Titanium dioxide (E171).
Black iron oxide (E172),
Appearance and package sizes of the medicine
Zavesca is a white 100 mg capsule with “OGT 918” printed in black on the end and “100” printed in black on the side.
A box contains 4 pressure packs with 21 capsules each, which gives a total of 84 capsules.
Marketing Authorisation Holder:
Janssen‑Cilag International NV
Turnhoutseweg 30
B‑2340 Beerse
Belgium
Manufacturer:
Janssen Pharmaceutica NV
Turnhoutseweg 30
B-2340 Beerse
Belgium