5 mg, 7.5 mg, 10 mg, 15 mg, 20 mg orodispersible tablets
olanzapine
What Zalasta is and what it is used for
Zalasta contains the active substance olanzapine. Zalasta belongs to the drug group neuroleptics and is used to treat the following conditions:
- Schizophrenia, is a disease with symptoms such as hearing, seeing, or sensing something that is not there, delusions, unusual suspiciousness, and withdrawal. People with these conditions may also feel depressed, anxious, or tense.
- Moderate to severe manic episodes, a condition with symptoms such as excitement and euphoria.
Zalasta prevents the recurrence of these symptoms in patients with bipolar disorder who have responded to olanzapine treatment in the manic phase.
What you need to know before you use Zalasta
Do not use Zalasta
- if you are allergic (hypersensitive) to olanzapine or any of the other ingredients of this medicine (listed in section 6). An allergic reaction can manifest itself as a skin rash, itching, swollen face, swollen lips, or difficulty breathing. If this should occur, contact your doctor.
- if you have previously had eye problems such as certain types of glaucoma (increased pressure in the eye).
Warnings and precautions
Talk to your doctor or pharmacist before taking Zalasta.
- The use of Zalasta in elderly patients with dementia is not recommended as it can cause serious side effects.
- Medicines of this type can cause abnormal movements of the face or tongue. Contact your doctor if this occurs.
- This type of drug can also cause a combination of fever, shortness of breath, sweating, muscle stiffness, and drowsiness. These side effects occur extremely rarely, but if they occur, contact your doctor immediately.
- Weight gain has occurred in patients taking Zalasta. You and your doctor should check your weight regularly. Consider referral to a dietitian or help with a diet list if necessary.
- High blood sugar and high blood fat levels ( triglycerides and cholesterol ) have occurred in patients taking Zalasta. Your doctor should do blood tests for blood sugar and fat levels before you start taking Zalasta and then at regular intervals during treatment.
- Tell your doctor if you or someone in your family has a history of blood clots, as medicines like these have been associated with blood clots.
You must tell your doctor if you suffer from any of the following diseases:
- stroke or mild form of stroke (temporary symptoms of stroke )
- Parkinson’s disease
- prostate problems
- irritable bowel (paralytic ileus )
- liver or kidney disease
- blood disease
- heart disease
- diabetes
- convulsions
- if you know you may have a salt deficiency as a result of prolonged severe diarrhea and vomiting or use diuretics ( diuretics )
For demented patients, the doctor must be informed if the patient has had a stroke or a milder form of stroke.
If you are over 65, your blood pressure should be checked regularly by your doctor.
Children and young people
Zalasta is not intended for patients under 18 years of age.
Other medicines and Zalasta
Only take other medicines during Zalasta treatment if advised by your doctor. Together with the following medicines, drowsiness can occur anti-depressants and anti-anxiety drugs, and sleeping aids (sedatives).
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
You must tell your doctor if you are taking:
- drugs against Parkinson’s disease
- carbamazepine (for epilepsy and mood stabilizer), fluvoxamine (for depression), or ciprofloxacin ( antibiotic ) – it may be necessary to adjust your Zalasta dose.
Zalasta with food, drink, and alcohol
Do not drink alcohol during treatment with Zalasta, because together with alcohol it can cause drowsiness.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine. You should not take this medicine if you are breastfeeding, as small amounts of Zalasta may pass into breast milk.
In newborn babies whose mothers have taken Zalasta during the last trimester (the last three months of pregnancy), the following symptoms may occur: tremors, stiff and/or weak muscles, sleepiness, agitation, breathing problems, and difficulty eating. If your child develops any of these symptoms, you may need to contact your doctor.
Driving ability and use of machinery
There is a risk that you may feel drowsy when using Zalasta. If this happens, do not drive or work with tools or machines and consult your doctor about this.
Zalasta contains aspartame
This medicine contains 0.50 mg of aspartame per 5 mg orodispersible tablet.
This medicine contains 0.75 mg of aspartame per 7.5 mg orodispersible tablet.
This medicine contains 1.00 mg of aspartame per 10 mg orodispersible tablet.
This medicine contains 1.50 mg of aspartame per 15 mg orodispersible tablet.
This medicine contains 2.00 mg of aspartame per 20 mg orodispersible tablet.
Aspartame is a source of phenylalanine. It can be harmful if you have phenylketonuria (PKU), a rare, inherited disease that leads to the accumulation of high levels of phenylalanine in the body.
How to use Zalasta
Always take this medicine as directed by your doctor. Ask your doctor or pharmacist if you are unsure.
The dose and duration of treatment are determined by your doctor. Dose one of Zalasta is 5 mg-20 mg per day.
Contact your doctor if symptoms return but do not stop taking the medicine unless your doctor tells you to.
You should take Zalasta once a day. Try to take Zalasta at the same time each day either with a meal or between meals.
Zalasta orally soluble tablets break easily and should therefore be handled carefully. Do not handle the tablet with wet hands as the tablet may dissolve. To remove the tablet from the package:
- Hold the edges of the blister and detach a blister cell from the rest of the blister by gently tearing it off along the perforation.
- Lift the edge of the foil and pull the foil off completely.
- Tip the tablet out into your hand.
- Place the tablet on the tongue immediately after removing it from the package.
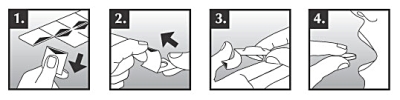
The tablet begins to dissolve in the mouth within seconds and can then be swallowed with or without water. Your mouth should be empty before placing the tablet on your tongue.
You can also put the tablet in a glass of water. Drink up immediately.
If you have used too much Zalasta
Patients who have taken too much Zalasta have experienced the following symptoms: fast heart rate, agitation/aggressiveness, difficulty speaking, involuntary movement disorders (especially of the face or tongue), and loss of consciousness. Other symptoms may be acute confusion, convulsions ( epilepsy ), coma, a combination of fever, shortness of breath, sweating, muscle stiffness, and drowsiness or sleepiness, slow breathing, difficulty breathing, high or low blood pressure, and abnormal heart rhythm. Contact your doctor or hospital immediately if you experience any of the listed symptoms. Take the remaining tablets with you.
If you forget to use Zalasta
Take your tablets as soon as you remember. Do not take two doses on the same day.
If you stop using Zalasta
It is important that you follow your doctor’s instructions and not stop taking the medicine because you feel better.
If you suddenly stop taking Zalasta, you may experience symptoms such as sweating, difficulty sleeping, tremors, anxiety or nausea, and vomiting. Your doctor may advise you to reduce the dose gradually before stopping treatment.
If you have further questions about this medicine, contact your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor immediately if you get:
- involuntary movement disorders (a common side effect that may affect up to 1 in 10 users) especially in the face or tongue
- blood clots in the veins (an uncommon side effect that may affect up to 1 in 100 people) especially in the legs (symptoms are swelling, pain, and redness of the legs). The blood clots can be transported to the lungs and cause chest pain and difficulty breathing. If you experience any of these symptoms, seek medical attention immediately.
- a combination of fever, faster breathing, sweating, muscle stiffness, and drowsiness or sleepiness (the frequency of this side effect cannot be estimated from the available data).
Very common side effects (which may affect more than 1 in 10 people) include weight gain, sleepiness, and increased levels of prolactin in the blood. At the beginning of treatment, some people may feel dizzy or faint (with a slow heart rate), especially when getting up from a lying or sitting position. This often goes away on its own. If not, contact your doctor.
Common side effects (may affect up to 1 in 10 users) include changes in levels of blood cells, blood lipids and, at the start of treatment, temporarily increased liver enzymes; increased sugar levels in the blood and urine; increased level of uric acid and creatinine phosphokinase in the blood; increased appetite; dizziness; restlessness; tremors; movement difficulties ( dyskinesia ); constipation; dry mouth; rash; impotence; extreme fatigue; fluid retention leading to swelling of the hands, ankles or feet; fever, joint pain and sexual problems such as reduced sex drive in men and women or erectile problems in men.
Uncommon side effects (may affect up to 1 in 100 users) include hypersensitivity (eg swelling of the mouth and throat, itching, rash); diabetes or worsening of diabetes, sometimes associated with ketoacidosis (ketones in the blood and urine) or coma; convulsions, generally in case of a known tendency to convulsions ( epilepsy ); muscle stiffness or spasm (including eye movements); crawling and feeling of restlessness in the legs when resting (restless legs); speech difficulties; stuttering; slow heartbeat; sun sensitivity; nosebleed; distended abdomen; drooling; memory loss or forgetfulness; urinary incontinence; difficulty urinating; hair loss; absent or shortened menstruation; and breast changes in men and women such as abnormal production of breast milk or abnormal enlargement.
Rare side effects (may affect up to 1 in 1,000 users) include lowering of the normal body temperature; abnormal heart rhythm; sudden, unexplained death; inflammation of the pancreas causing severe stomach pain, fever, and malaise; liver disease manifesting as yellowing of the skin and whites of the eyes; muscle disease manifesting in unexplained aches and pains; and prolonged and/or painful erection.
Very rare side effects include serious allergic reactions such as drug reactions with eosinophilia and systemic symptoms (DRESS). DRESS presents initially with flu-like symptoms with a rash on the face and then by more widespread rash, fever, enlarged lymph nodes, elevated levels of liver enzymes seen in blood tests, and elevated levels of a type of white blood cell ( eosinophils ).
When taking medication with olanzapine, elderly patients with dementia may experience a stroke, pneumonia, urinary incontinence, increased tendency to fall, extreme fatigue, visual hallucinations, increased body temperature, skin redness, and difficulty walking. Some deaths have been reported in this specific patient group.
For patients with Parkinson’s disease, Zalasta may worsen symptoms.
How to store Zalasta
Keep this medicine out of the sight and reach of children.
Use before the expiry date stated on the packaging. The expiration date is the last day of the specified month.
Store in the original packaging. Light sensitive. Moisture sensitive. No special temperature instructions.
Medicines must not be thrown into the drain or among the household waste. Ask the pharmacist how to deal with medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
- The active substance is olanzapine. Each orodispersible tablet contains 5 mg, 7.5 mg, 10 mg, 15 mg or 20 mg olanzapine.
- Other ingredients are mannitol, microcrystalline cellulose, crospovidone, low-substituted hydroxypropyl cellulose, aspartame, calcium silicate, and magnesium stearate.
Appearance and package sizes of the medicine
Zalasta orodispersible tablets 5 mg, 7.5 mg, 10 mg, 15 mg, and 20 mg are round, slightly biconvex, yellow-marbled tablets with random dots.
Zalasta 5 mg, 7.5 mg, 10 mg, 15 mg, and 20 mg orodispersible tablets are supplied in cartons of 14, 28, 35, 56, or 70 tablets in blisters.
Marketing Authorisation Holder
KRKA, dd, Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
Manufacturer
KRKA, dd, Novo mesto, Šmarješka cesta 6, 8501 Novo mesto, Slovenia
KRKA-POLSKA Sp. z o. o., ul. Równoległa 5, 02-235 Warsaw, Poland
TAD Pharma GmbH, Heinz-Lohmann-Straße 5, 27472 Cuxhaven, Germany