tablets 600 mg and oral suspension 120 mg/ml
Felbamate
What Taloxa is and what it is used for
What Taloxa is
Antiepileptic drugs.
What Taloxa is used for
Taloxa is used against Lennox-Gastaut syndrome and other types of epilepsy that cannot be controlled with other drug treatments, in adults and children over 4 years of age.
Taloxa tablets and oral suspension can be used together with other medicines against epilepsy.
What you need to know before taking Taloxa
Do not take Taloxa:
- if you are allergic to felbamate or any of the other ingredients of this medicine (listed in section 6).
- if you previously had problems with the blood (e.g. anemia, low blood cell count, problems with bleeding, easy bruising, frequent infections ).
- if you have had problems with the liver, eg jaundice (yellow whites of the eyes or skin) or hepatitis.
Warnings and precautions
- Seek medical attention immediately if you develop a rash, fever, swelling of the nose, eyes, or throat if you have breathing problems.
- If you have been taking Taloxa or any other medicine regularly to control seizures, do not stop taking your medicine without your doctor’s advice.
- Drink plenty of water during treatment with Taloxa to avoid kidney stones in the urine and to ensure that the medicine disappears from the body.
- A small number of people treated with anti- epileptic drugs such as Taloxa have had thoughts of harming themselves or committing suicide. If you ever have these thoughts, contact your doctor immediately.
- If you are a woman of childbearing age, you must use an effective method of contraception during treatment and for up to 1 month after the end of treatment. Treatment with felbamate may reduce the effectiveness of oral contraceptives, therefore another effective method should be used.
Taloxa tablets and oral suspension have been associated with a few cases of life-threatening disorders of the blood and liver function. Inform your doctor if you have previously had problems with your blood or liver before you start taking Taloxa tablets or suspension . Contact your doctor if you experience unusual symptoms such as bleeding, bruising, frequent infections , fatigue, jaundice (yellow whites of the eyes or yellow skin), weight loss, vomiting or abdominal pain.
Blood samples
While you are taking this medicine, your doctor will decide that blood tests are taken frequently (every two weeks) to check your condition.
Other medicines and Taloxa
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines. You may be taking other medicines for epilepsy . The dose of one of these medicines may need to be increased or decreased while you are taking Taloxa.
If you take Taloxa together with other antiepileptic drugs , you may notice increased side effects . Contact a doctor if side effects become bothersome.
The effect of birth control pills can be affected. Consult the doctor if you use birth control pills .
Taloxa with food and drink
You can take Taloxa with meals.
Pregnancy and breastfeeding
Taloxa should not be used during pregnancy, unless absolutely necessary. If you are a woman of childbearing age, you must use an effective contraceptive method during the treatment and up to 1 month after the end of the treatment. Treatment with felbamate may reduce the effectiveness of oral contraceptives, therefore another effective method should be used.
If you are pregnant or planning to become pregnant, consult a doctor immediately. You should not stop taking your medicine until you have discussed this with your doctor. Your doctor may decide to change your treatment.
Do not breast-feed if you are taking Taloxa. Felbamat is excreted in breast milk. It can harm your child, especially causing blood or liver damage.
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Driving ability and use of machinery
Treatment with Taloxa may make you tired or dizzy. When Taloxa is given to children and young people, it should be taken into account that dizziness and fatigue can also occur when you are in traffic (e.g. cycling). You yourself are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects . Description of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. Discuss with your doctor or pharmacist if you are unsure.
Taloxa contains
Tablets
Taloxa tablets contain lactose . If you have an intolerance to some sugars, you should consult your doctor before taking this medicine.
Taloxa tablets contain less than 1 mmol (23 mg) of sodium per dose , i.e. are almost “sodium-free”.
Oral suspension
Taloxa oral suspension contains methyl and propyl parahydroxybenzoates which may cause allergic reactions in some people (possibly delayed).
Taloxa oral suspension contains 1.05 g of sorbitol per filled dosing syringe (5.0 ml) corresponding to 210 mg/ml. Sorbitol is a source of fructose. If you (or your child) have an intolerance to certain sugars, or if you (or your child) have been diagnosed with hereditary fructose intolerance , a rare, inherited disease that makes it impossible to break down fructose, consult a doctor before using this medicine. Sorbitol can cause stomach/intestinal discomfort and can have a mild laxative effect.
Taloxa oral suspension contains less than 1 mmol (23 mg) of sodium per 5.0 ml, i.e. it is almost “sodium-free”.
Taloxa oral suspension contains 10 mg sodium benzoate per 5 ml corresponding to 2 mg/ml.
How to take Taloxa
Always take this medicine as directed by your doctor. Ask your doctor or pharmacist if you are unsure.
How large a dose of Taloxa you should take
It is common for Taloxa tablets or oral suspension to be taken 2 (every 12 hours) or 3 (every 8 hours) times per day. The correct dose and dosing interval are determined by the doctor. During the first weeks of treatment, the dose may need to be adjusted by the doctor. Take the medicine at the same time every day. Do not skip a dose or take more than the prescribed dose .
How to take your dose of Taloxa oral suspension
- shake the bottle before each dose
- dose one should be taken with the dosing syringe provided in the package
1. Instructions for use:
Measure dose one into the supplied 5.0 ml dosing syringe.
The plastic dosing syringe consists of two parts, an opaque cylinder and a white plunger that fits into the cylinder.
The flask is graduated in 0.1 ml increments starting at 0.5 ml (top of the flask) and ending at 5 ml.
A. Insert the dosing syringe into the bottle of Taloxa oral suspension .
B. Keep the tip in the liquid while pulling out the plunger. As the syringe fills with the solution, you will see the numbers on the plunger move up.
C. Pull up the plunger until you can see the correct number of ml for the dose you are measuring.
D. Remove the syringe from the bottle and check that the correct amount is displayed at the bottom of the syringe. If you have too much or too little, try again until you have measured the correct amount.
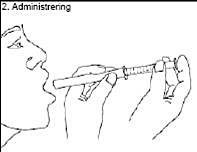
2. Administration:
Hold the syringe in your mouth and take one dose by pushing down on the plunger.
Swallow dose one.
3. Cleaning:
Disassemble the syringe and rinse it with water after each use.
If you or your child has taken too much Taloxa
If you have ingested too much medicine or if, for example, a child has ingested the medicine by mistake, immediately contact a doctor or hospital for an assessment of the risk and advice.
If you or your child forget to take Taloxa
Take the missed dose as soon as possible if it is not soon time for the next dose . Do not take a double dose to make up for a missed dose . It is important to return to the normal dosing schedule as soon as possible.
If you or your child stops taking Taloxa
Do not stop taking this medicine unless your doctor tells you to. Abrupt discontinuation of anti- epileptic medication , including Taloxa, may increase the risk of seizures.
If you or your child have further questions about this medicine, contact your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects , although not everybody gets them.
Taloxa tablets and oral suspension have been associated with cases of serious disorders of the blood (rare), liver function (very rare) and hypersensitivity reactions (rare), which may be life-threatening, see section “Warnings and precautions”.
Contact a doctor immediately if you get :
- any unusual symptoms while taking Taloxa, such as bleeding, bruising, frequent infections , tiredness, jaundice (yellow whites of the eyes or yellow-colored skin), weight loss, vomiting, or abdominal pain.
- blisters in the mouth, nose or eyes or blisters and peeling of the skin, muscle or joint pain, fever, skin rash.
Common side effects (affects more than 1 in 100 users but less than 1 in 10 users) :
- Weight loss, loss of appetite
- Insomnia, drowsiness, disturbances in coordination of muscle movements, dizziness, headache
- Fatigue
- Double vision, abnormal vision
- Nausea, vomiting, upset stomach, abdominal pain, diarrhea.
Uncommon side effects (affects less than 1 in 100 users but more than 1 in 1,000 users) :
- Hypophosphatemia (abnormally low levels of phosphate in the blood)
- Speech disorders, depression, stiffness, anxiety
- Rash
- Abnormal gait.
Rare side effects (affects less than 1 in 1,000 users but more than 1 in 10,000 users) :
- Serious blood count changes, which include thrombocytopenia (decreased number of platelets ), leukopenia (decreased number of white blood cells ), neutropenia (decreased number of white blood cells ) and anemia (decreased number of red blood cells ). There can be combinations of these, which include a reduction of all three types together and an inability of the bone marrow to produce all three types of cells.
- Increased number of seizures (convulsions)
- Allergic shock (severe allergic reaction throughout the body)
- Severe hypersensitivity reaction such as: Stevens Johnson syndrome, skin blisters, toxic epidermal necrolysis (blisters in the mouth, nose, eyes and other mucous membranes, blisters and peeling of the skin)
- Muscle or joint pain
- Fever.
Very rare side effects (affects less than 1 user in 10,000) :
- Constipation
- Crystals in the urine
- Liver problems that can be serious and can include fatal liver failure.
Additional side effects in children and adolescents
A similar side effect pattern exists for children. In addition, upper respiratory tract infections have been frequently observed in children, although the relationship with the treatment is not likely.
How Taloxa should be stored
Keep this medicine out of the sight and reach of children.
Store at a maximum of 25 °C.
Do not use this medicine without consulting a doctor or pharmacist if you notice changes in the appearance of Taloxa.
Use before the expiry date:
Tablets: on the box Ugd.dat and on the printed packaging EXP.
Oral suspension: on the carton and on the label Issue date.
The expiration date is the last day of the specified month.
After first opening the bottle
Close bottle well. Suspension one is stable for 3 months after opening the bottle.
Medicines must not be thrown into the drain or among household waste. Ask the pharmacist how to dispose of medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
- The active substance is defective.
- Other ingredients for tablets are pregelatinized starch , microcrystalline cellulose, croscarmellose sodium (see section 2 “Taloxa contains”), lactose monohydrate (see section 2 “Taloxa contains”), magnesium stearate.
- Other ingredients for oral suspension are: sorbitol (E420) (see section 2 “Taloxa contains”), glycerol (E422), microcrystalline cellulose + carmellose sodium, simethicone emulsion, saccharin sodium, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216) (see section 2 “Taloxa contains”), sodium benzoate (E211) (see section 2 “Taloxa contains”), polysorbate 80, Prosweet “G”#859 ( glycerol (E422), vanillin, ethyl maltol) and purified water.
Appearance and package sizes of the medicine
Taloxa 600 mg tablets
Taloxa 600 mg tablets are white capsule-shaped scored tablets with the SP logo and 600 on one side.
Pressed packaging with 100 tablets.
Taloxa oral suspension
Taloxa 120 mg/ml oral suspension (white to off-white opaque suspension )
Glass bottle: 230 ml
A 5 ml dosing syringe, CE marked, for oral use is included
Marketing Authorisation Holder:
NV Organon
Kloosterstraat 6
5349 AB Us
Netherlands
Manufacturer:
Schering-Plough Labo NV
Industriepark 30
B-2220 Heist-op-de-Berg
Belgium