0.15 mg/dose nasal spray, solution
buserelin
What Suprecur is and what it is used for
Suprecur contains a medicine called buserelin. Buserelin is similar to a naturally occurring hormone released by the brain. It belongs to a group of medicines called “luteinizing hormone-releasing hormone analogues” (LHRH analogues ).
Suprecur is used to reduce the growth of endometriosis and to relieve the symptoms, e.g. pain. Suprecur is also used for pretreatment before in vitro fertilization ( in vitro fertilization).
In endometriosis, Suprecur works by inhibiting the function of the ovaries, which leads to a decrease in the production of the female sex hormones estradiol and progesterone. Endometriosis, which results from the endometrium being found incorrectly outside the uterus, is normally stimulated by the female sex hormones estradiol and progesterone. As Suprecur inhibits the ovaries, the treatment means that the growth of endometriosis also decreases. Another effect of Suprecur inhibiting the function of the ovaries is the absence of periods.
In test tube fertilization ( in vitro fertilization), the inhibition of ovarian function is used to avoid spontaneous ovulation. When the concentration of estradiol has fallen to a low level, treatment with gonadotropin , a hormone that stimulates ovulation, is started.
The buserelin contained in Suprecur may also be approved to treat other conditions not mentioned in this product information. Ask your doctor, pharmacist or other healthcare professional if you have any further questions and always follow their instructions.
What you need to know before using Suprecur
Do not use Suprecur
- If you are allergic to buserelin or any of the other LHRH analogues (e.g. leuprolide, goserelin, triptorelin) or any other ingredient in this medicine (listed in section 6).
- If you are pregnant.
Warnings and precautions
Talk to your doctor or pharmacist before using Suprecur:
- If you have high blood pressure . Your doctor or nurse should regularly check your blood pressure as blood pressure can be affected by Suprecur.
- If you have any cardiovascular disease including heart rhythm disturbance ( arrhythmia ), or are being treated with medicines for this. The risk of suffering from heart rhythm disorders may be increased when using Suprecur.
- If you have diabetes . Check your blood sugar levels regularly. This is because Suprecur can affect your metabolism and thus your blood sugar levels.
- If you have ever had depression. You should be vigilant about your mental health because there is a risk that your depression may return or worsen. Talk to your doctor if you feel down/depressed while taking Suprecur.
Suprecur can cause a decrease in bone density, osteoporosis (weakening of the skeleton) and an increased risk of bone fractures, especially if you have risk factors for osteoporosis such as alcohol abuse, are smokers, have a family history of osteoporosis or if you have been treated with anti- epileptic drugs for a long time or with cortisone . The risk of bone fracture increases with the length of the treatment period. Your doctor should measure your bone density regularly to be able to prescribe preventive measures against osteoporosis if necessary.
For symptom relief of endometriosis
- Before treatment of endometriosis begins, use of birth control pills must be discontinued. When using Suprecur, you cannot become pregnant as long as the inhibition of ovarian function remains. During the treatment period, it is recommended that non-hormonal contraceptives (e.g. condoms) be used.
- Treatment with Suprecur will lead to missed periods. It is an expected effect of the treatment. Inform your doctor if you continue to get your period during treatment.
- While you are being treated with Suprecur, you are not expected to become pregnant. If treatment is interrupted, if only for a few days, ovulation and pregnancy may occur. If pregnancy occurs, treatment with Suprecur must be stopped immediately and the doctor informed.
- A decrease in bone density may occur (weakening of the skeleton), which is why the duration of treatment should not exceed 6 months. Repeated periods of treatment should only be undertaken after careful assessment by the attending physician.
During pretreatment for in vitro fertilization
- When Suprecur is used at the same time as another LHRH analogue , there is an increased risk of having overstimulated ovaries (hyperstimulation syndrome, OHSS), especially if you have polycystic ovary syndrome(enlarged ovaries filled with follicles). Signs of overstimulated ovaries may include abdominal discomfort, abdominal pain, abdominal distention, nausea, vomiting, ovarian cysts, ovarian enlargement, shortness of breath or shortness of breath, diarrhea, low urine output. Concentration of the blood, i.e. reduced amount of fluid in the blood, which gives an increased concentration of the blood’s components. Increased risk of blood clot formation. Conditions in the ovaries that can lead to severe pain in the lower abdomen. Serious blood clotting side effects can also occur. The outcome can be fatal. Each stimulation cycle must therefore be closely followed by a doctor to detect this early.
- Before starting treatment with Suprecur, it is recommended that a pregnancy test be performed to rule out pregnancy.
Children and young people
As there is a lack of information on the drug’s safety and effectiveness in children, Suprecur should not be used in children.
Other medicines and Suprecur
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Suprecur can affect or be affected by certain other medicines.
In particular, inform the doctor if you are using:
- drugs for diabetes . This is because Suprecur can reduce the effect of these medicines.
- medicines to treat heart rhythm disorders (eg quinidine , procainamide , amiodarone and sotalol).
- other medicines that may increase the risk of heart rhythm disturbances when used together with Suprecur, e.g. methadone (used as a pain reliever and in the treatment of drug addiction), moxifloxacin (an antibiotic ), antipsychotics (used in severe mental illnesses).
Pregnancy and breastfeeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before using this medicine.
Pregnancy: Suprecur should not be used during pregnancy. If you become pregnant, discontinue treatment and consult a doctor.
Breastfeeding: Suprecur passes into breast milk in small amounts. No negative effects on the breast-fed baby have been seen, but it is still recommended that breast-feeding be avoided during treatment with Suprecur.
Driving ability and use of machinery
Suprecur can cause side effects (such as dizziness) that can affect your concentration and reaction speed. You yourself are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects . Description of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. Discuss with your doctor or pharmacist if you are unsure.
Suprecur contains sodium and benzalkonium chloride
Suprecur contains sodium
This medicine contains less than 1 mmol (23 mg) of sodium per ml, i.e. it is almost ‘sodium-free’.
Suprecur contains benzalkonium chloride
This medicine contains 0.1 mg of benzalkonium chloride per ml of nasal spray, solution. Benzalkonium chloride can cause irritation and swelling inside the nose, especially with prolonged use of the medicine.
How to use Suprecur
Always use this medicine as directed by your doctor or pharmacist. Ask your doctor or pharmacist if you are unsure.
Dosage
instructions Symptom relief in endometriosis
In order to quickly achieve an effect and to avoid treatment being given during an already started pregnancy, treatment should always be started on the first or second day of menstruation.
The normal daily dose is 0.9 mg, divided into three doses. A spray dose of 0.15 mg is taken in each nostril morning, noon and evening. Other times can be chosen provided that the dosing occasions are evenly distributed throughout the day.
One bottle of 10 ml (= 100 doses) is sufficient for 2 weeks of treatment (0.9 mg/day) and must then be discarded.
As pre-treatment for in vitro fertilization
Different treatment schedules are applied for in vitro fertilization. The treatment can either start on the first or second day of menstruation or about a week before the next expected period, but in the latter case pregnancy must be ruled out.
The normal daily dose is 0.6 mg, divided into four doses. A spray dose of 0.15 mg is taken in one nostril 4 times a day, preferably at the following times: 7 am, 12 pm, 5 pm and 10 pm.
Some patients need a higher dose . If you are prescribed 1.2 mg of Suprecur per day, follow the schedule above, but spray one dose into each nostril at each dosing time.
One bottle of 10 ml (= 100 doses) is sufficient for 3 weeks of treatment (0.6 mg/day), or 1.5 weeks (1.2 mg/day) and must then be discarded.
If you have used too much Suprecur
If you have ingested too much medicine or if, for example, a child has accidentally ingested the medicine, contact a doctor or hospital for an assessment of the risk and advice.
Too much of this medicine can make you feel lightheaded, headache, restlessness, hot flashes, dizziness, nausea, stomach pain, swelling ( edema ) of the ankles, and breast tenderness.
If you have any further questions about this medicine, contact your doctor, nurse or pharmacist.
Instructions
for use Assembling the nasal spray
1. Glass bottle: Unscrew the cap.
2. Plastic container: Take out the dosing pump.
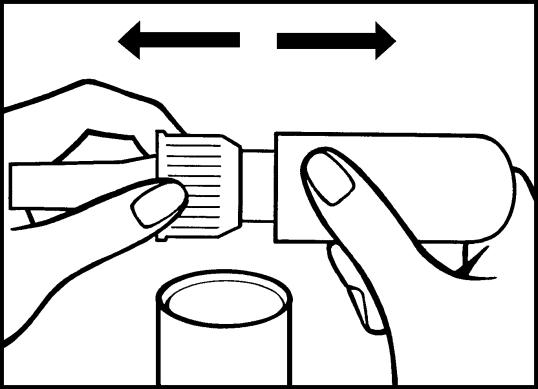
a) Remove the protective cap (white) from the dosing pump.
2 a
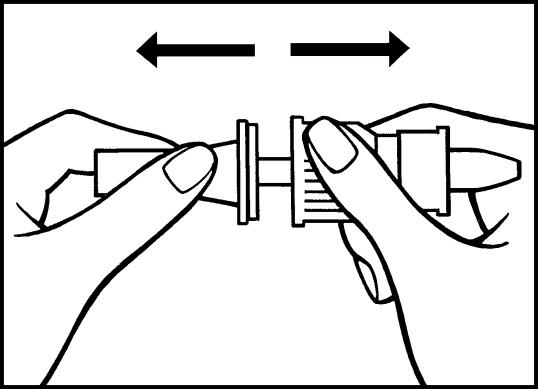
b) Then remove the semi-transparent protective cap from the pump tube.
2 b
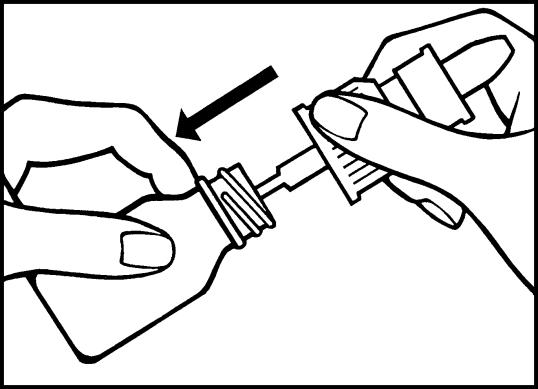
3. Insert the pump tube into the bottle and screw on the dosing pump. The nasal spray is now fully assembled.
3
4. Before using the spray bottle for the first time, pump about 5-8 times into the air until an even “cloud” is obtained. The spray should be held upright. Further test spraying is not necessary but only results in consumption of the preparation.
Use
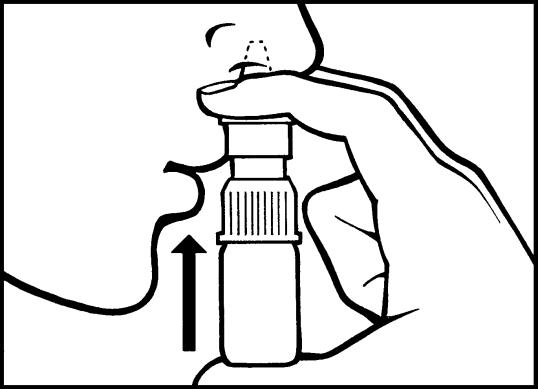
5. When the spray is to be used, the head is slightly bent forward and the spray is held vertically.
Dosage: See Dosage Instructions.
5
ATTENTION! Make sure the nose is free of mucus when you have a cold. The preparation is then effective even in case of nasal congestion.
After use, the dosing pump must remain screwed onto the glass bottle. Put on the white protective cap and store the spray upright.
Possible side effects
Like all medicines, this medicine can cause side effects , although not everybody gets them.
Contact a doctor immediately if you develop symptoms such as a rash, difficulty swallowing or breathing, swelling of the lips, face, throat or tongue. This could be a serious allergic reaction .
Reported side effects ar
Common (may affect up to 1 in 10 users)
- Weight gain or weight loss
- Palpitation
- Headache, drowsiness, dizziness, fatigue
- Odor and taste change
- Nausea, vomiting, diarrhoea, constipation, abdominal pain
- Dry skin, acne , skin rashes
- Increased or decreased head hair
- Pain in the back, arms, legs and joints, muscle stiffness in the shoulder
- Feeling of heat in the skin, sweating
- Irritation of the nasal mucosa, nosebleeds
- Period-like bleeding, vaginal dryness, vaginal discharge, ovarian cysts (abnormal cyst formation in the ovary), breast tenderness, change in breast size, pain during intercourse
- Sleep disturbances, decreased sex drive, nervousness, mood changes, depression (with long-term use).
Uncommon (may affect up to 1 in 100 users)
- Influence on liver function values
- Memory and concentration disorders, crawling and tingling in the skin
- Dry eyes, visual disturbance (for example, blurred vision), feeling of pressure behind the eyes
- Cracked nails, increased or decreased body hair
- Increased thirst, change in appetite
- Fluid accumulation ( edema ) in the face, arms and legs
- Hypersensitivity reaction (redness, itching , hives )
- Milk discharge, inflammation of the vagina
- Mood changes, depression (with short-term use), anxiety.
Rare (may affect up to 1 in 1,000 users)
- Altered blood fat values
- Tinnitus, hearing impairment
- Increased blood pressure in patients with already high blood pressure
- Shortness of breath, spasm in the musculature of the trachea
- Severe allergic reactions with shock
- Emotional instability, anxiety.
Very rare (may affect up to 1 in 10,000 users)
- Changes in the blood picture
- Discomfort and pain in muscles and bones
- Decreased glucose tolerance (can lead to impaired diabetic control in diabetics)
- Growth of benign tumor in pituitary gland
- Deterioration of general well-being.
Has been reported (occurring in an unknown number of users):
- Changes ( QT prolongation) that can be seen on the electrocardiogram ( ECG ).
The side effects of Suprecur are dose-dependent, i.e. when Suprecur is used in low doses , such as during pretreatment for in vitro fertilization ( in vitro fertilization), these side effects occur less frequently and are less pronounced than when treating endometriosis .
How to store Suprecur
Store at a maximum of 25 ºC.
Do not freeze.
Keep this medicine out of the sight and reach of children.
Use before the expiry date which is stated on the carton after EXP. The expiration date is the last day of the specified month.
Opened bottle must be used within 5 weeks.
Medicines must not be thrown into the drain or among household waste. Ask the pharmacist how to dispose of medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
- The active substance is buserelin. 1 g of nasal spray contains buserelin acetate corresponding to 1.5 mg buserelin. 1 dose of nasal spray corresponds to 0.15 mg buserelin.
- Other ingredients are citric acid monohydrate, sodium citrate dihydrate, sodium chloride, benzalkonium chloride ( preservative ), water for injections.
Appearance and package sizes of the medicine
One package contains 2 brown glass bottles of 10 ml clear solution and 2 dosing pumps.
Marketing Authorisation Holder
CHEPLAPHARM Arzneimittel GmbH
Ziegelhof 24
17489 Greifswald
Germany
Manufacturer
Sanofi-Aventis Deutschland GmbH
Industriepark Höchst
Brüningstraße 50
65926 Frankfurt am Main
Germany