10 mg prolonged-release tablet is
oxycodone hydrochloride
What Oxikodon Depot Acino is and what it is used for
Oxycodone Depot Acino prolonged-release tablets are a centrally acting, strong painkiller from the opioid group.
Oxycodone Depot Acino prolonged-release tablets are used to treat severe pain which can only be treated satisfactorily with opioid painkillers.
Oxycodone hydrochloride contained in Oxycodone Depot Acino prolonged-release tablets may also be approved for the treatment of other conditions not mentioned in this leaflet. Ask your doctor, pharmacist, or another healthcare professional if you have any further questions, and always follow their instructions.
What you need to know before taking Oxycodone Deopt Acino prolonged-release tablets
Do not take Oxycodone Depot Acino prolonged-release tablets
- if you are allergic to oxycodone or any of the other ingredients of this medicine, (listed in section 6)
- if you suffer from severe respiratory distress ( respiratory depression ) with too little oxygen in the blood ( hypoxia ) and/or too much carbon dioxide in the blood,
- if you suffer from severe chronic obstructive pulmonary disease, cor pulmonale (heart changes due to constant congestion of the circulation in the lungs) or, severe bronchial asthma,
- if you suffer from intestinal obstruction (paralytic ileus ),
- if you have severe abdominal pain or suffer from delayed emptying of the stomach,
Warnings and cautions
Talk to your doctor or pharmacist before taking Oxikodon Depot Acino prolonged-release tablets
- if you are elderly or debilitated,
- if your lung, liver, or kidney function is severely impaired,
- if you suffer from myxedema (a certain disease of the thyroid gland), impaired function of the thyroid gland,
- if you suffer from insufficient adrenal function ( Addison’s disease ),
- if you suffer from prostate hypertrophy,
- if you suffer from adrenal insufficiency,
- if you suffer from alcoholism or undergo alcohol withdrawal,
- if you suffer from a known opioid addiction,
- if you suffer from inflammation of the pancreas (pancreatitis),
- in conditions with increased pressure in the brain, (eg after head injury)
- if you suffer from disorders of the regulation of blood circulation,
- if you suffer from colic in the bile ducts and ureter,
- if you suffer from inflammatory bowel disease,
- if you have low blood pressure, ( hypotension )
- if you suffer from decreased blood volume, ( hypovolemia )
- if you suffer from epilepsy or tend to have seizures,
- if you are taking MAOIs (to treat depression).
Oxycodone should be used with caution in patients who have undergone abdominal surgery as opiates are known to reduce bowel motility.
Oxycodone Depot Acino prolonged-release tablets are not recommended before or within 12-24 hours after surgery.
Oxycodone Depot Acino prolonged-release tablets have a primary dependence potential. When used for a long time, tolerance to the effect and gradually higher doses may be required to maintain control over the pain.
Chronic use of Oxikodon Depot Acino prolonged-release tablets can lead to physical dependence and withdrawal syndrome may occur with abrupt discontinuation of treatment. These symptoms may include yawning, enlarged pupils, increased tear production, runny nose, tremors, increased sweating, anxiety, upset, twitching, and insomnia. When a patient no longer needs oxycodone treatment, it is advisable to reduce the dose gradually to prevent withdrawal symptoms.
When used as directed, in patients with chronic pain, the risk of developing physical or mental dependence is significantly reduced and needs to be weighed against the potential benefits. Discuss this with your doctor.
If you drink alcohol while taking Oxikodon Depot Acino, it may make you feel more sleepy or increase the risk of serious side effects such as shallow breathing with a risk of respiratory arrest and unconsciousness. You should not drink alcohol while taking Oxycodone Depot Acino.
The prolonged-release tablets should not be given to patients with known alcohol and drug abuse.
Children and young people
Oxycodone Depot Acino is not recommended for use in children and adolescents under 18 years of age.
Elderly patients
In elderly patients, dose adjustment is usually not necessary.
Other medicines and Oxycodone Depot Acino
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
- Medicines that suppress the activity of the central nervous system (eg sleeping pills or sedatives [sedatives, hypnotics ], other medicines that affect the nervous system [phenothiazines, neuroleptics ], medicines used to treat allergies or vomiting [antihistamines, antiemetics ], and other opioids or alcohol may aggravate the side effects of oxycodone, in particular respiratory depression ( respiratory depression ).
- Drugs with anticholinergic effects (eg drugs that suppress psychosis [neuroleptics], drugs used to treat allergies [antihistamines] or vomiting [antiemetics], drugs used to treat Parkinson’s disease ) may aggravate some of the side effects of oxycodone ( eg constipation, dry mouth or urinary disorders) Cimetidine may inhibit the breakdown of oxycodone. The effects of other drugs that may have a significant effect on the degradation of oxycodone have not been studied.
- Monoamine oxidase inhibitors (MAOIs) may potentiate the side effects of oxycodone (eg, stimulation of increased or decreased blood pressure ). Oxycodone should be used with caution in patients taking or have taken MAOIs in the last 2 weeks
- In individual cases, more or less extensive blood clot formation has been observed when anticoagulant drugs (coumarin-type anticoagulants) are taken with Oxycodone Depot Acino prolonged-release tablets.
Concomitant use of the following drugs may lead to reduced degradation of oxycodone in the body, which may lead to increased blood concentrations and the effects of oxycodone:
- Medicines for the treatment of infections caused by bacteria, so-called macrolide antibiotics (eg clarithromycin, erythromycin, and telithromycin)
- Medicines for the treatment of fungal infections (eg ketoconazole , voriconazole, itraconazole and posaconazole)
- Drugs called protease inhibitors and used to treat HIV (eg boceprevir, ritonavir, indinavir, nelfinavir, and saquinavir).
- The antidepressant drug paroxetine
- The antiarrhythmic drug quinidine and
- Grapefruit juice
Dose one of oxycodone may need to be adjusted during concomitant treatment.
The following drugs may increase the breakdown of oxycodone in the body, which may lead to a reduction in the effects of oxycodone:
- Rifampicin ( antibiotic )
- Carbamazepine and phenytoin ( antiepileptic drugs )
- St. John’s wort (natural remedy)
Dose one of oxycodone may need to be adjusted during concomitant treatment.
Oxycodone Depot Acino with food, drink, and alcohol
Alcohol intensifies alertness and reactivity and may potentiate side effects such as drowsiness and shortness of breath.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Do not use Oxycodone Depot Acino prolonged-release tablets if you are pregnant. There are limited data from the use of oxycodone in pregnant women.
Oxycodone passes through the placenta and into the baby’s bloodstream.
Long-term use of oxycodone during pregnancy may cause withdrawal symptoms in newborns. The use of oxycodone during childbirth may cause respiratory depression in newborns.
Breast-feeding
Do not use Oxycodone Depot Acino prolonged-release tablets while breastfeeding as oxycodone passes into breast milk and may cause respiratory depression in newborns.
Driving and using machines
Oxycodone can impair alertness and responsiveness to such an extent that the ability to drive and use machines is affected or ceases altogether. To see the side effects that affect motor skills and concentration, see section 4 (Possible side effects ).
In stable treatment, a general ban on driving is not necessary. The attending physician must assess the individual situation. Discuss with your doctor if and under what circumstances you can drive a vehicle.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires sharpened vigilance. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. Descriptions of these effects and side effects can be found in other sections. Therefore, read all the information in this leaflet
guidance. If you are not sure, talk to your doctor or pharmacist.
Oxycodone Depot Acino contains excipients
This medicine contains sugar (sucrose). If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
How to take Oxycodone Depot Acino prolonged-release tablets
Always take this medicine exactly as your doctor has told you. Ask your doctor or pharmacist if you are unsure.
The recommended dose is:
Oxycodone Depot Acino 10 mg prolonged-release tablets:
Adults (over 18 years)
The usual starting dose is 10 mg oxycodone hydrochloride (1 prolonged-release tablet ) at 12-hour intervals. However, your doctor will prescribe the dose needed to treat the pain.
Further determination of the daily dose one, division into single doses, and any adjustments during the further treatment is performed by the attending physician and depends on the previous dose one.
Patients who have previously taken opioids can start treatment with higher doses, taking into account their previous experience with opioid treatment.
Some patients taking Oxikodon Depot Acino 10 mg prolonged-release tablets according to a fixed schedule need fast-acting pain relief when needed to control breakthrough pain. Oxycodone Acino 10 mg prolonged-release tablets are not intended for the treatment of acute pain and/or breakthrough pain.
To treat non-cancerous pain, a dose of 2 prolonged-release tablets twice daily (40 mg oxycodone hydrochloride daily) is usually sufficient, but a higher dose may be necessary.
Patients with cancer pain usually require a dose of 80 mg to 120 mg of oxycodone hydrochloride, which can be increased to 400 mg in individual cases.
For higher doses, Oxikodon Depot Acino 20 mg, 40 mg, and 80 mg prolonged-release tablets are available.
The treatment needs to be checked regularly about pain relief and other effects to achieve the best possible pain treatment and to be able to treat any side effects that occur in good time and to decide whether the treatment should continue.
At-risk patients
If you have impaired kidney and/or liver function, your doctor may prescribe a lower starting dose.
Oxycodone Depot Acino prolonged-release tablets must not be taken with alcoholic beverages.
Method of administration and treatment time
The prolonged-release tablets should be swallowed (whole or divided) with a sufficient amount of liquid (½ glass of water) with or without food in the morning and the evening according to a fixed schedule (eg 8.00 in the morning and 20.00 in the evening).
The prolonged-release tablets must not be crushed or chewed as this will lead to rapid release of oxycodone due to damage to the sustained release properties. Ingestion of chewed or crushed Oxycodone Depot Acino prolonged-release tablets leads to rapid release and rapid uptake of a potentially lethal dose of oxycodone (see section “If you have taken too much Oxycodone Depot Acino prolonged-release tablets” ).
Oxycodone Depot Acino prolonged-release tablets should be swallowed orally. When used for injection ( injection into a vein), the tablet’s excipients (especially talc) can lead to death ( necrosis ) of local tissue, changes in the lungs (granulomas in the lungs), or other serious, possibly fatal consequences.
Instructions for opening the blister:
This medicine is supplied in a child-resistant package. The film-coated tablets cannot be squeezed through the blister. Follow these instructions when opening the blister.
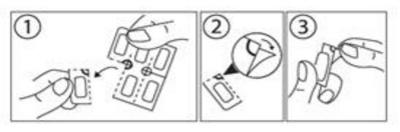
Subtract a dose by tearing the blister at the perforated line.
2. An unsealed part of the blister, where the perforated lines meet, then becomes available.
3. Peel off the foil on the unsealed part of the blister.
Your doctor will adjust your dosage depending on how severe the pain is and how the treatment works for you. Take the number of prolonged-release tablets you have been prescribed by your doctor twice a day.
If you take more Oxikodon Depot Acino prolonged-release tablets then you should
If you have ingested too much medicine or if e.g. a child ingested the medicine accidentally contact a doctor or hospital immediately for risk assessment and advice.
The following symptoms may occur constricted pupils ( miosis ), impaired breathing ( respiratory depression ), skeletal muscle weakness, and a drop in blood pressure. In severe cases, circulatory collapse, mental and physical inactivity (dormancy), unconsciousness ( coma ), decrease in heart rate, and accumulation of water in the lungs (non-cardiogenic edema ) may occur; abuse of high doses of strong opioids such as oxycodone can be fatal. Under no circumstances should you be exposed to situations that require increased concentration, such as driving a car.
If you forget to take Oxikodon Depot Acino prolonged-release tablets
If you have taken a smaller dose of Oxikodon Depot Acino prolonged-release tablet than instructed or if you miss taking tablets, the pain relief will be insufficient or stop completely.
You can make up for a missed tablet if the next regular intake is not to take place until at least 8 hours. You can then continue to take tablets as instructed,
You can also take a prolonged-release tablet if the time to the next regular intake is shorter, but postpone the next intake by 8 hours. In principle, you should not take Oxikodon Depot Acino prolonged-release tablets more than once every 8 hours.
Do not take a double dose to make up for a forgotten tablet.
If you stop taking Oxikodon prolonged-release Acino prolonged-release tablets
Do not stop treatment without informing your doctor. When a patient no longer needs to be treated with Oxikodon Depot Acino prolonged-release tablets, it is advisable to reduce the daily dose gradually to avoid withdrawal symptoms. If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Important serious side effects or symptoms that you should be aware of and actions to take when these side effects or symptoms occur:
If you get any of the following side effects, stop taking Oxikodon Depot Acino prolonged-release tablets and contact your doctor immediately.
Respiratory depression is the most significant risk posed by opioids and is most likely to occur in elderly or debilitated patients. As a result, opioids can cause a sharp drop in blood pressure in patients prone to low blood pressure.
In addition to this, oxycodone can cause constricted pupils, tracheal spasms, and spasms in the smooth muscles and dampen the cough reflex.
Other possible side effects are
Very common (may affect more than 1 in 10 people):
- Drowsiness dizziness; headache
- constipation; nausea; vomiting
- itching
Common (may affect up to 1 in 10 people):
- Loss of appetite, anxiety, depression, changes in activation (usually sedation, sometimes followed by drowsiness), nervousness, sleep disturbances, difficulty with the thought process, confusion
- involuntary muscle contractions (shaking)
- bronchial spasms (difficulty breathing or wheezing), shortness of breath ( dyspnoea )
- dry mouth, stomach pain, diarrhea, stomach upset (acid regurgitation)
- skin rash, increased sweating
- a feeling of weakness ( asthenia )
Uncommon (may affect up to 1 in 100 people):
- Hypersensitivity reactions (allergic reactions)
- Increased secretion of antidiuretic hormone ( ADH ), less water in the body (dehydration)
- Upset, emotional instability, euphoria, decreased sex drive, drug abuse
- Memory loss ( amnesia ), seizures (cramps), increased muscle tension, involuntary muscle twitching, decreased sensitivity to touch ( hypesthesia ), language disturbance, fainting, tingling ( paresthesia ), taste changes ( dysgeusia )
- Changes in tear secretion, visual impairment, pupillary contraction.
- Dizziness ( vertigo ), acute increased sensitivity to sound (hyperacusis)
- Awareness of heartbeat (in connection with withdrawal syndrome)
- dilated blood vessels ( vasodilation )
- Increased cough; inflammation of the pharynx; Suva; voice changes, respiratory depression
- Gallkolik; sores in the mouth; gingivitis, inflammation of the mouth ( stomatitis ), difficulty swallowing ( dysphagia ), flatulence, belching, blockage in the intestine ( ileus )
- Increased liver enzymes
- Dry skin, herpes simplex
- Difficulty urinating ( urinary retention )
- Disorder of sexual function
- Injuries due to accidents, pain (eg chest pain), migraines, tremors, withdrawal syndrome, malaise, swelling of tissue due to fluid retention ( edema ), tolerance, thirst
Rare (may affect up to 1 in 1,000 people):
- Lymph node disease ( lymphadenopathy )
- Seizures, especially in patients with epilepsy or patients with a tendency to seizures
- Low blood pressure, especially when standing up
- Hives
- Blood in the urine ( hematuria )
- Changes in body weight (increase or decrease), cellulite
No known frequency (can not be calculated from the available data)
- Severe hypersensitivity reactions (anaphylactic reactions)
- Aggression
- Increased sensitivity to pain
- Bleeding in the gums, caries, increased appetite, dark stools
- Blockage of the bile ducts, sometimes painful (cholestasis, biliary colic )
- Absence of menstrual bleeding ( amenorrhea )
Countervailing measures
If you notice any of the side effects described above, your doctor will take appropriate action.
Constipation side effects can be prevented by a high-fiber diet and increased water intake.
If you suffer from nausea or vomiting, your doctor will prescribe the appropriate medicine.
How to store Oxikodon Depot Acino
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the blister / can and on the carton after “EXP”. The expiration date is the last day of the specified month.
Do not store above 30 ° C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the pack and other information
Content declaration
Oxycodone Depot Acino contains 10 mg prolonged-release tablets
– The active substance is oxycodone hydrochloride. Each prolonged-release tablet contains 10 mg oxycodone hydrochloride equivalent to 9 mg oxycodone.
– The other ingredients are:
Tablet core: Sugar spheres (sucrose, maize starch), hypromellose, macrogol 6000, talc, ethylcellulose, hydroxypropyl cellulose, propylene glycol, magnesium stearate, microcrystalline cellulose, powdered cellulose, silica colloidal anhydrous.
Tablet coating: Hypromellose, talc, macrogol 6000, titanium dioxide (E171), brown iron oxide (E 172), red iron oxide (E 172).
What the medicine looks like and the contents of the pack
Oxycodone Depot Acino 10 mg prolonged-release tablet your appearance and pack sizes
Oxycodone Depot Acino 10 mg prolonged-release tablets are brown-red, biconvex, oblong prolonged-release tablets with a scoreline on both sides.
Oxycodone Depot Acino 10 mg prolonged-release tablets are available in blister packs, with child-resistant closure, if 10, 14, 20, 25, 28, 30, 40, 50, 56, 60, 98 and 100 prolonged-release tablets and in HDPE jars, with PP child-resistant twist-off cap, if 14, 20, 25, 28, 30, 50, 56, 98, 100 and 200 prolonged-release tablets .
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorisation Holder:
Acino AG
Am Windfeld 35
83714 Miesbach
Germany
Manufacturer:
Orion Corporation, Orion Pharma
Orionintie 1
FI-02200 Espoo
Finland
For further information on this medicine, please contact your local representative:
Orion Pharma AB, Danderyd
medinfo@orionpharma.com
This medicinal product is authorized under the European Economic Area under the names:
Denmark Oxycodone hydrochloride “Acino” 10 mg
Norway Zacrenoxin depot 10 mg
Poland Oxycodone Acino 10 mg
Slovakia Oxycodone Acino 10 mg
Germany Oxycodone- HCl -Acino / 10 mg