3 mg / g gel
brimonidine
What Mirvaso is and what it is used for
Mirvaso contains the active substance brimonidine, which belongs to a group of medicines commonly referred to as “alpha-agonists”.
Mirvaso is used on the skin of the face to treat redness due to rosacea in adult patients.
Redness of the face due to rosacea is caused by heavy blood flow in the skin of the face, which is due to enlargement (enlargement) of the small blood vessels in the skin.
When Mirvaso is used, it works by narrowing these blood vessels again, which reduces the excess blood flow and redness.
What you need to know before using Mirvaso
Do not use Mirvaso
- if you are allergic to brimonidine or any of the other ingredients of this medicine (listed in section 6).
- to children under 2 years, as they may be at greater risk of side effects from the drugs that are absorbed through the skin.
- if you are taking certain medicines used to treat depression or Parkinson’s disease , including so-called monoamine oxidase inhibitors (MAOIs, eg selegiline , or moclobemide), tricyclic antidepressants (such as imipramine) or tetracyclic antidepressants (such as maprotiline, mianserin or mirtazapine). Using Mirvaso while you are taking these medicines may cause your blood pressure to drop .
Warnings and cautions
Talk to your doctor or pharmacist before using Mirvaso in particular about:
- the skin of your face is irritated or has open sores.
- you have problems with your heart or blood circulation.
- you have depression, decreased blood flow to the brain or heart, decreased blood pressure when standing, decreased blood flow in the hands, feet or skin, or Sjögren’s syndrome (a chronic disease in which the body’s natural defenses – the immune system – attack the glands that produce moisture).
- you have or have previously had kidney or liver problems.
- you have undergone or are planning to undergo a laser treatment of the skin of your face.
It is important to start treatment with a small amount of gel, increase the dose gradually but do not exceed the maximum dose of 1 gram (about 5 pea-sized clicks). See also the instructions in “How to use Mirvaso”.
Do not apply Mirvaso more than once a day and do not exceed the maximum daily dose of 1 gram (approximately 5 pea-sized clicks). See also the instructions in “How to use Mirvaso”.
Exacerbated skin redness, the sudden feeling of heat often with flushing or a burning sensation in the skin
Up to 1 in 6 patients experience that their redness is worse when it returns compared to before treatment. This usually occurs during the first two weeks of treatment and usually disappears if treatment is stopped, in most cases within a few days. Before resuming treatment with Mirvaso topical gel, test it on a small area of the face for a day when you have the opportunity to stay at home. If you do not experience a worsening of redness or burning sensation, you can continue with the usual treatment (see section 3).
If you experience an aggravated redness or if an unexpected redness occurs, stop treatment and consult a doctor.
Talk to your doctor if any of the above apply to you, as this medicine may not be suitable for you.
Children and young people
Do not give this medicine to children or adolescents under 18 years of age as the safety and efficacy have not been established for this age group. This is especially important for children under 2 years of age (see “Do not use Mirvaso”).
Other medicines and Mirvaso
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, as these medicines may affect your treatment with Mirvaso, or Mirvaso may affect your treatment with these medicines.
Do not use Mirvaso with selegiline, moclobemide, imipramine, mianserin, or maprotiline, which are medicines that can be used for depression or Parkinson’s disease, as this may lead to changes in the effectiveness of Mirvaso or may increase the risk of side effects such as a drop in blood pressure. Mirvaso).
Also, tell your doctor if you are taking any of the following medicines:
- medicines used to treat pain, sleep disorders or anxiety disorders
- medicines used to treat mental disorders (chlorpromazine), used for hyperactivity (methylphenidate) or used for high blood pressure (reserpine).
- drugs that act in the same way in the body as Mirvaso (other alpha – agonists , eg clonidine; so-called alpha-blockers or alpha- antagonists , eg prazosin, isoprenaline , which are commonly used to treat high blood pressure , slow heartbeat or asthma ).
- cardiac glycosides (eg digoxin ) used to treat heart problems.
- antihypertensive drugs such as beta- blockers or calcium channel blockers (eg propranolol, amlodipine).
Talk to your doctor if any of the above apply to you, or if you are unsure.
Mirvaso with alcohol
Tell your doctor if you drink alcohol regularly as it may affect your treatment with this medicine.
Pregnancy and breastfeeding
The use of Mirvaso is not recommended during pregnancy. This is because its effects on your unborn baby are unknown. You should not use this medicine while breastfeeding, as it is not known if this medicine passes into breast milk.
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine.
Driving and using machines
Mirvaso has no significant effect on the ability to drive and use machines.
Mirvaso contains:
- methyl parahydroxybenzoate (E218) which may cause allergic reactions (possibly delayed).
- Propylene glycol (E1520) which may cause skin irritation.
How to use Mirvaso
Always use this medicine exactly as your doctor has told you. If you are not sure, talk to your doctor or pharmacist.
Important: Mirvaso is for adults only and use on the skin of the face only. Do not use this medicine on other parts of the body, especially moist body surfaces such as the eyes, mouth, nose, or vagina.
Do not swallow.
Keep Mirvaso Gel out of the reach of children.
How to use Mirvaso
Mirvaso is recommended to be used on the face once a day.
Start treatment with a small amount of gel (a pea-sized click) during the first week, as shown by your doctor or nurse.
If your symptoms persist or only improve slightly, you can gradually increase the amount of gel after the first week. Spread it evenly and evenly in a very thin layer in the way that your doctor or nurse has shown you. You mustn’t take more than the maximum daily dose of 1 gram (5 pea-sized clicks applied to the entire face).
Wash hands immediately after the application of this medicine.
If your symptoms worsen during treatment with Mirvaso (increased redness or burning sensation), stop treatment and consult a doctor – see also section 2 “Warnings and precautions”.
You need to avoid eyes, eyelids, lips, mouth, and the inside of the nose. If a gel ends up in any of these areas, wash it off immediately with plenty of water. If you experience an aggravated redness or burning sensation, stop using Mirvaso and consult a doctor if necessary.
Do not apply any other dermatological or cosmetics immediately before the daily application of Mirvaso. You should use such products only after applying Mirvaso has dried.
Be careful when opening the tube/pump for the first time so that you do not spill more gel than necessary. If this happens, discard the excess gel so that you do not apply more than the recommended dose. See the section “How to use Mirvaso” above
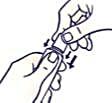
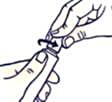
To open the tube with a child-resistant lid:
To avoid spillage, do not squeeze the tube while opening or closing it.
Push the lid down and turn counterclockwise (turn left). Then pull off the lid.
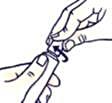
To close the tube with a child-resistant lid:
Press down and turn clockwise (turn right).
If you use more Mirvaso than you should
If you use more than the maximum daily dose of 1 gram within 24 hours, it may lead to skin irritation or other side effects at the application site. Repeated doses within the same 24-hour period can lead to side effects such as low blood pressure, drowsiness, or fatigue.
Contact your doctor for advice on what to do.
If someone, especially a child, accidentally swallows Mirvaso, it may cause serious side effects that need to be treated in a hospital.
Contact a doctor immediately or see an emergency room immediately if you, a child, or someone else swallows this medicine and has any of these symptoms: feeling dizzy from low blood pressure, vomiting, tiredness or drowsiness, weakened or irregular heartbeat, narrow pupils ( pupil contraction ), difficulty breathing or slow breathing, lethargy, low body temperature and cramps (seizures). Bring the medicine pack so that the doctor can see what the person has swallowed.
If you forget to use Mirvaso
Mirvaso works daily, starting on the first day of treatment. Therefore, if you miss a daily dose, redness will not decrease that day. Do not use a double dose to make up for a forgotten dose and continue your treatment as prescribed.
If you stop using Mirvaso
A possible consequence that can occur if the treatment is stopped before the treatment regimen is completely completed is that the disease returns to its original state.
Contact your doctor before ending your treatment, who can advise you on a suitable replacement treatment if necessary.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you get less common side effects like severe irritation or inflammation of the skin, rash, pain or discomfort in the skin, dry skin, feeling hot in the skin, tingling or swelling or swollen face, or common side effects such as aggravated rosacea, discontinue treatment and contact your doctor as this medicine may not be suitable for you. In some cases, the symptoms may extend beyond the skin area being treated. See also section 2 “Warnings and precautions”.
If you get a contact allergy (eg an allergic reaction, rash), or rare angioedema (a rare severe allergic reaction, usually with swelling of the face, mouth, or tongue), stop using Mirvaso and see a doctor immediately.
Mirvaso can also cause the following other side effects:
Common side effects (may affect up to 1 in 10 people):
- sudden feeling of heat often with flushing of the skin
- noticeable pallor where gel is applied
- redness of the skin, burning sensation in the skin or itching
Uncommon side effects (may affect up to 1 in 100 people):
- acne
- dry mouth
- feeling of cold in hands and feet
- feeling of warmth
- headache
- nasal congestion
- swollen eyelids
- hives
- dizziness
Rare side effects (may affect up to 1 in 1,000 people):
- lowered blood pressure
- decreased heart rate (slow heart rate called bradycardia ).
How to store Mirvaso
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, tube, and pump after EXP. The expiration date is the last day of the specified month.
No special storage instructions.
Do not freeze.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Contents of the pack and other information
Content declaration
- The active substance is brimonidine. One gram of gel contains 3.3 mg of brimonidine, which is equivalent to 5 mg of brimonidine tartrate.
- The other ingredients are carbomer, methyl parahydroxybenzoate (E218), phenoxyethanol, glycerol , titanium dioxide, propylene glycol (E1520), sodium hydroxide and purified water. See section 2 for information on methyl parahydroxybenzoate and propylene glycol.
What the medicine looks like and contents of the pack
Mirvaso is a white to light yellow, opaque gel. It is provided in tubes containing 2, 10, or 30 grams of gel, or in a non-ventilated pump system containing 30 g of gel.
Pack size 1 tube or 1 pump.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorisation Holder
Galderma International
Tour Europlaza, 20 avenue André Prothin – La Défense 4
La Cedex Defense 92927
France
Manufacturer
Galderma Laboratories
ZI Montdésir
74540 Alby sur Chéran
France
or
Galderma Laboratorium GmbH
Toulouser Allee 19a-23a
D-40211 Düsseldorf
Germany
Contact the representative of the marketing authorization holder to find out more about this medicine:
Galderma Nordic AB
Tel: + 46 18 444 0330
e-mail: nordic@galderma.com