| Exelon 4.6 mg / 24 h transdermal patch |
| Exelon 9.5 mg / 24 h transdermal patch |
| Exelon 13.3 mg / 24 h transdermal patch |
| rivastigmine |
1. What Exelon is and what it is used for
The active substance in Exelon is rivastigmine.
Rivastigmine belongs to a class of substances called cholinesterase inhibitors. In patients with Alzheimer’s dementia, certain nerve cells in the brain die. This leads to low levels of the neurotransmitter acetylcholine (a substance that allows nerve cells to communicate with each other). Rivastigmine works by blocking the enzymes that break down acetylcholine ( acetylcholinesterase and butyrylcholinesterase). By blocking these enzymes, Exelon increases the levels of acetylcholine in the brain, thus helping to reduce the symptoms of Alzheimer’s disease.
Exelon is used to treat adult patients with mild to moderate Alzheimer’s dementia, a progressive brain disease that gradually affects memory, intellectual ability, and behavior.
2. What you need to know before using Exelon
Do not use Exelon
- if you are allergic to rivastigmine (the active substance in Exelon) or any of the other ingredients of this medicine (listed in section 6).
- if you have ever had an allergic reaction to a similar type of medicine (carbamate derivatives).
- if you get a skin reaction that spreads outside the size of the patch, if a more intense local reaction occurs (eg blisters, increasing skin inflammation, swelling) and if it does not improve within 48 hours after removal of the transdermal patch.
If this applies to you, talk to your doctor and do not use Exelon transdermal patches.
Warnings and cautions
Talk to your doctor before using Exelon:
- if you have or have ever had, irregular or slow heartbeats.
- if you have or have ever had, an active stomach ulcer.
- if you have or have ever had, difficulty urinating.
- if you have or have ever had seizures.
- if you have or have ever had, asthma or severe respiratory problems.
- if you suffer from tremors.
- if you have low body weight.
- if you get reactions from the stomach or intestines such as. nausea, vomiting, and diarrhea. You may become dehydrated (lose too much fluid) if vomiting or diarrhea persists for a long time.
- if you have impaired liver function.
If any of the above applies to you, your doctor may need to check you more closely when you are being treated with this medicine.
If you have not used a patch for more than three days, do not put on a new one until you have talked to your doctor.
Children and young people
There is no relevant use of Exelon for the pediatric population in the treatment of Alzheimer’s disease.
Other medicines and Exelon
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
Exelon may affect other anticholinergic medicines, some of which are medicines for stomach cramps or seizures (eg dicyclomine), medicines to treat Parkinson’s disease(eg amantadine), or medicines to prevent motion sickness (eg diphenhydramine, scopolamine, meclozine).
Exelon Patches should not be given concomitantly with metoclopramide (a medicine used to relieve or prevent nausea or vomiting). Taking both medicines at the same time can cause problems such as stiff joints or tremors in the hands.
If you are going to have surgery while using Exelon transdermal patches, tell your doctor that you are using the medicine, as Exelon transdermal patches may intensify the effects of certain muscle relaxants during anesthesia.
Caution should be exercised when taking Exelon Plaster at the same time as beta-blockers (medicines such as atenolol for high blood pressure, angina, and other heart conditions). Taking both medicines at the same time can cause problems such as slower heartbeat ( bradycardia ), which can lead to fainting or unconsciousness.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
If you are pregnant, the benefits of using Exelon must be weighed against the possible effects on your unborn baby. Exelon should not be used during pregnancy unless clearly necessary.
You should not breast-feed while you are being treated with Exelon transdermal patches.
Driving and using machines
Your doctor will tell you if you can drive and use machines safely when you have this disease. Exelon transdermal patches can cause fainting spells or you may feel very confused. If you feel dizzy or confused, do not drive, use machines or perform any other tasks that require your attention.
3. How to use Exelon
Always use Exelon transdermal patches exactly as your doctor has told you. If you are not sure, talk to your doctor, pharmacist, or nurse.
IMPORTANT:
- Remove the previous day’s patches before putting ONE new patch.
- Use only one patch per day.
- Do not cut the patch into pieces.
- The patch should be pressed firmly with the palm of your hand for at least 30 seconds.
How to start treatment
Your doctor will tell you which Exelon transdermal patch is most suitable for you.
- Treatment is usually started with Exelon 4.6 mg / 24 hours.
- The usual recommended daily dose is Exelon 9.5 mg / 24 hours per day. If this dose is well-tolerated, your doctor may increase the dose to Exelon 13.3 mg / 24 hours.
- Use only one Exelon patch at a time and replace the patch with a new one after 24 hours.
During treatment, your doctor may need to adjust the dose to adjust to your individual needs.
If you have not used a patch for more than three days, do not put it on the next patch until you have talked to your doctor. The treatment with transdermal patches can be resumed with the same dose if the treatment has only been interrupted for a maximum of three days. Otherwise, your doctor will restart your treatment with Exelon 4.6 mg / 24 hours.
Exelon can be used with food, drink, and alcohol.
Where should your Exelon transdermal patch be affixed?
- Before applying the patch, make sure the skin is scarred, dry, and hair-free, free of powder, oil, moisturizer, or lotion, which may cause the patch to not adhere properly to the skin, free from scratches, rashes, and/or irritation.
- If you are already using patches, remove what you are wearing before applying a new one. If you have multiple patches simultaneously attached to the body, you can get too high a dose of the drug, which can potentially be dangerous.
- Apply ONE patch per day in ONLY ONE of the possible locations shown in the following diagram:
- left upper arm or right upper arm
- the left or right side of the upper part of the chest (avoid the breasts themselves)
- the left or right side of the upper back
- the left or right side of the lower back
| Remove the previous day’s patch after 24 hours before putting ONE new patch in ONLY ONE of the following possible places. |
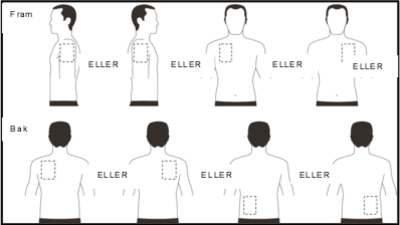
When changing patches, you must remove the previous day’s patches before applying the new patch to a new spot on the skin each time (eg on the right side of the body one day, on the left side the next day, on the upper part of the body one day, on the lower part of the body the next day). Do not put a new patch on the same skin surface twice within 14 days.
How should your Exelon transdermal patch be affixed?
Exelon patches are thin, opaque plastic patches that are attached to the skin. Each patch is sealed in a patch envelope, which protects it until it is put on. Do not open the patch envelope or remove the patch until just before you apply it.
The patch should be used at all times until it is time to change to a new one. Feel free to try to find different places to attach the patches, places that feel comfortable for you, and where the clothes do not rub against the patch.
How to remove your Exelon transdermal patch?
Gently grasp one edge of the patch and slowly pull it away from the skin. If adhesive residue remains on the skin, moisten the area with warm water and mild soap, or use baby oil to remove them. Alcohol or other solvents (nail polish removers or other solvents) should not be used.
You should wash your hands with soap and water when the patch has been removed. If the patch comes in contact with the eyes, or if the eyes turn red after handling the patch, rinse immediately with plenty of water and consult a doctor if symptoms do not go away.
Can you use your Exelon transdermal patch when bathing, swimming, or sunbathing?
- Bathing, swimming, or showering should not affect the patch. Make sure that the patch does not come off on such occasions.
- Do not expose the patch to external heat sources (eg excessive sunbathing, sauna, solarium) for a long time.
What to do if Exelon transdermal patches fall off?
If a patch falls off, apply a new one for the rest of the day, then change to a new one again at the usual time the next day.
When and for how long should you use your Exelon transdermal patch?
- To benefit from your treatment, you must apply a new patch every day, preferably at the same time each day.
- Use only one patch at a time and replace the patch with a new one after 24 hours.
If you use more Exelon then you should
If you accidentally put on more than one patch, remove all the patches from your skin and tell your doctor that you accidentally put on more than one patch. You may need medical attention. Some people, who have inadvertently used too much Exelon, have felt nauseous, vomited, or had diarrhea, high blood pressure, and hallucinations. Slow heart rate and fainting spells can also occur.
If you forget to use Exelon
If you find that you have forgotten to apply a patch, apply a new one immediately. You can apply the next patch at the usual time the next day. Do not apply two patches to compensate for a forgotten patch.
If you stop using Exelon
If you stop using the patch, talk to your doctor or pharmacist.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, Exelon transdermal patches can cause side effects, although not everybody gets them.
You will likely experience some side effects, especially when you start treatment or when the dose is increased. Usually, the side effects gradually disappear when the body gets used to the drug.
Remove the patch and contact your doctor immediately if you get any of the following side effects that could be serious:
Common (may affect up to 1 in 10 people)
- Loss of appetite
- Dizziness
- The feeling of agitation or tiredness
- Urinary incontinence (inability to retain urine)
Uncommon (may affect up to 1 in 100 people)
- Heart rhythm disorders (both fast and slow heartbeats)
- Seeing things that are not real (hallucinations)
- Gastric ulcer
- Dehydration (fluid loss)
- Hyperactivity (high activity, restlessness)
- Aggressiveness
Rare (may affect up to 1 in 1,000 people)
- Case
Very rare (may affect up to 1 in 10,000 people)
- Stiffness in arms or legs
- Shaking hands
No known frequency (frequency can not be calculated from available data)
- An allergic reaction where the patch has been applied, such as blisters or inflammation of the skin
- Worsening of the symptoms of Parkinson’s disease – such as trembling, stiffness, sluggish gait
- Inflammation of the pancreas (symptoms include severe pain in the upper abdomen, usually with nausea and vomiting)
- Fast or irregular heartbeat
- High blood pressure
- Cramps (seizures)
- Disorders of liver function (yellowing of the skin, yellowing of the whites of the eyes, abnormally dark urine or unexplained nausea, unexplained vomiting, fatigue, and loss of appetite)
- Elevated liver values
- Feeling of restlessness
- Nightmares
Remove the patch and contact your doctor immediately if you get any of the above side effects.
Additional side effects that have been seen with Exelon capsules or oral solution and that may occur with the patch:
Common (may affect up to 1 in 10 people)
- Too much saliva
- Decreased appetite
- Restlessness
- General malaise
- Trembling or feeling of confusion
- Increased sweating
Uncommon (may affect up to 1 in 100 people)
- Irregular heartbeat (eg fast heartbeat)
- Difficulty sleeping
- Accidentally fall
Rare (may affect up to 1 in 1,000 people)
- Cramps (seizures)
- Intestinal ulcers
- Chest pain – can be caused by a heart attack
Very rare (may affect up to 1 in 10,000 people)
- High blood pressure
- Inflammation of the pancreas (signs of it include severe pain in the upper abdomen, usually with nausea and vomiting)
- Gastrointestinal bleeding (manifests as blood in the stool or case of vomiting)
- Seeing things that are not real (hallucinations)
- Some people who have had severe vomiting have had stretch marks in the esophagus
5. How to store Exelon
- Keep this medicine out of the sight and reach of children.
- Do not use this medicine after the expiry date which is stated on the carton and patch envelope after EXP. The expiration date is the last day of the specified month.
- Do not store above 25 ° C.
- Keep the transdermal patch in the patch envelope until use.
- Do not use the patch if you see that it is damaged or if the patch envelope shows signs of opening.
- After removing the patch, fold it twice with the adhesive side inwards and press together. Put the used patch back in the plaster envelope and throw it away so that children cannot get hold of it. Do not poke in the eyes with your fingers and wash your hands with soap and water after removing the patch. If your home municipality burns household waste, you can dispose of the patch together with the household waste. Otherwise, return used patches to the pharmacy, preferably in the original packaging.
6. Contents of the packaging and other information
Content declaration
- The active substance is rivastigmine.
- Exelon 4.6 mg / 24 h: Each patch that releases 4.6 mg rivastigmine per 24 hours is 5 cm 2 and contains 9 mg rivastigmine.
- Exelon 9.5 mg / 24 hours: Each patch that releases 9.5 mg of rivastigmine per 24 hours is 10 cm 2 and contains 18 mg of rivastigmine.
- Exelon 13.3 mg / 24 hours: Each patch that releases 13.3 mg of rivastigmine per 24 hours is 15 cm 2 and contains 27 mg of rivastigmine.
- The other ingredients are lacquered polyethylene terephthalate film, alpha-tocopherol, poly (butyl methacrylate, methyl methacrylate), acrylic copolymer, silicone oil, dimethicone, polyester film coated with a fluoropolymer.
What the medicine looks like and the contents of the pack
Each transdermal patch is thin and consists of three layers. The outside is beige and marked with one of the following:
- “Exelon”, “4.6 mg / 24 h” and “AMCX”,
- “Exelon”, “9.5 mg / 24 h” and “BHDI”,
- “Exelon”, “13.3 mg / 24 h” and “CNFU”.
Each transdermal patch is sealed in a patch envelope.
Exelon 4.6 mg / 24 hours transdermal patches and Exelon 9.5 mg / 24 hours transdermal patches are available in packs of 7, 30, or 42 transdermal patches and multi-packs of 60, 84, or 90 transdermal patches .
Exelon 13.3 mg / 24 hours transdermal patches are available in packs of 7 or 30 transdermal patches and multi-packs of 60 or 90 transdermal patches.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Novartis Europharm Limited
Vista Building
Elm Park, Merrion Road
Dublin 4
Ireland
Manufacturer
Novartis Pharma GmbH
Roonstraße 25
D-90429 Nuremberg
Germany
Novartis Pharmacéutica, SA
Ronda de Santa Maria 158
08210 Barberà del Vallès, Barcelona
Spain
Contact the representative of the marketing authorization holder to find out more about this medicine:
| Belgium / Belgique / BelgienNovartis Pharma NVTel: +32 2 246 16 11 | LithuaniaSIA Novartis Baltics Lithuanian branchesTel: +370 5 269 16 50 |
| BulgariaNovartis Bulgaria EOODTel: +359 2 489 98 28 | Luxembourg / LuxemburgNovartis Pharma NVTel: +32 2 246 16 11 |
| Czech RepublicNovartis sroTel: +420 225 775 111 | HungaryNovartis Hungary Kft.Tel .: +36 1 457 65 00 |
| DenmarkNovartis Healthcare A / STel: +45 39 16 84 00 | MaltaNovartis Pharma Services Inc.Tel: +356 2122 2872 |
| GermanyNovartis Pharma GmbHTel: +49 911 273 0 | The NetherlandsNovartis Pharma BVTel: +31 88 04 52 111 |
| EestiSIA Novartis Baltics Eesti subsidiaryTel: +372 66 30 810 | NorwayNovartis Norge ASTel: +47 23 05 20 00 |
| GreeceNovartis (Hellas) AEBE:Ηλ: +30 210 281 17 12 | AustriaNovartis Pharma GmbHTel: +43 1 86 6570 |
| SpainNovartis Pharmacéutica, SATel: +34 93 306 42 00 | PolandNovartis Poland Sp. z ooTel .: +48 22 375 4888 |
| FranceNovartis Pharma SASTel: +33 1 55 47 66 00 | PortugalNovartis Farma – Pharmaceutical Products, SATel: +351 21 000 8600 |
| CroatiaNovartis Hrvatska dooTel. +385 1 6274 220 | RomaniaNovartis Pharma Services Romania SRLTel: +40 21 31299 01 |
| IrelandNovartis Ireland LimitedTel: +353 1 260 12 55 | SloveniaNovartis Pharma Services Inc.Tel: +386 1 300 75 50 |
| IcelandVistor hf.Phone: +354 535 7000 | Slovak RepublicNovartis Slovakia sroTel: +421 2 5542 5439 |
| ItalyNovartis Farma SpATel: +39 02 96 54 1 | Finland / FinlandNovartis Finland OyPuh / Tel: +358 (0) 10 6133 200 |
| LatviaSIA Novartis BalticsTel: +371 67 887 070 | United KingdomNovartis Pharmaceuticals UK Ltd.Tel: +44 1276 698370 |
This leaflet was last modified
2020-11-19