250 IU, 500 IU, 1000 IU, 1500 IU, 2000 IU, 3000 IU powder and solvent for injection , solution
nonacog alpha ( recombinant coagulation factor IX)
1. What BeneFIX is and what it is used for
BeneFIX is an injectable product containing coagulation factor IX produced by recombinant DNA technology. The active substance in BeneFIX is nonacog alfa. People born with haemophilia B (Christmas disease) do not have enough coagulation factor IX to control bleeding. BeneFIX works by replacing factor IX in hemophilia B patients to allow their blood to coagulate.
BeneFIX is used for treating and preventing bleeding in patients with haemophilia B ( congenital haemorrhagic disease I coagulation factor IX) in all age groups.
2. What you need to know before using BeneFIX
Do not use BeneFIX
- if you are allergic to nonacog alfa or any of the other ingredients of this medicine (listed in section 6).
- if you are allergic to hamster protein.
Warnings and cautions
- Talk to your doctor or pharmacist before using BeneFIX.
- Contact your doctor immediately if the bleeding does not stop as expected.
- Allergic reactions are possible. The product may contain traces of hamster proteins (see “Do not use BeneFIX”). Potentially life-threatening anaphylactic reactions (severe allergic reaction ) have occurred with factor IX products, including BeneFIX. Early signs of allergic reactions include difficulty breathing, shortness of breath, swelling, hives that may be widespread throughout the body, itching , chest tightness, wheezing, low blood pressure , blurred vision and anaphylaxis (severe allergic reaction that may cause difficulty swallowing and / or or breathing, redness or swelling of the face and / or hands).
- If allergic or anaphylactic reactions occur, discontinue injection of BeneFIX immediately and consult a physician , or seek emergency medical attention immediately . If severe allergic reactions occur, alternative therapy should be considered.
- It is less common for previously treated patients receiving factor IX products to develop neutralizing antibodies (inhibitors). However, during treatment with BeneFIX, you should be closely monitored to check if factor IX inhibitors have been developed. The same applies to treatment with all products containing factor IX.
- In the literature, it has been reported that there is an association between the presence of factor IX inhibitors and allergic reactions. Therefore, if you get allergic reactions such as those described above, you should be investigated for the presence of inhibitors. It should be noted that patients taking factor IX inhibitors may be at higher risk of anaphylaxis during additional BeneFIX treatment sessions.
- The body’s production of factor IX is regulated by the factor IX gene. Patients who have specific mutations on the factor IX gene, such as larger deletion mutations, may be more likely to develop factor IX inhibitors and / or have allergic reactions. If you are known to have such a mutation, your doctor may monitor you extra closely for signs of an allergic reaction, especially when you start treatment with BeneFIX for the first time.
- Due to the risk of allergic reactions to factor IX, your first injections of BeneFIX should be given under medical supervision so that appropriate medical treatment for allergic reactions is available.
- Even in the absence of inhibitors, a higher dose of BeneFIX may be needed compared to other plasma IX products that you may have taken before. Careful monitoring of factor IX plasma activity (which measures your blood’s ability to coagulate) must therefore be performed to adjust dose one. If the bleeding is not under control after the recommended dose , consult your doctor.
- If you have liver or heart disease or if you have recently undergone surgical treatment, there is an increased risk of clot formation (coagulation complications).
- Renal impairment ( nephrotic syndrome ) has been reported at high doses of factor IX produced by blood plasma in haemophilia B patients with factor IX inhibitors who have previously experienced allergic reactions.
- Sufficient data have not been obtained from clinical trials regarding BeneFIX treatment in patients who have not previously received any treatment (patients who have never received a factor IX infusion before ).
- It is recommended that you note the name and batch number of the product each time you use BeneFIX. You can use one of the removable labels on the bottle to document the batch number in your diary or to report side effects .
Other medicines and BeneFIX
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Pregnancy, breastfeeding, and fertility
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, you should only take BeneFIX as prescribed by your doctor. It is not known whether BeneFIX can cause harm to an unborn baby when taken by pregnant women. Your doctor may advise you to stop taking BeneFIX if you are breast-feeding or pregnant.
Ask your doctor or pharmacist for advice before taking this medicine.
Driving and using machines
BeneFIX has no effect on the ability to drive or use machines.
BeneFIX contains sodium
After preparation BeneFIX contains 0.2 mmol (4.6 mg) of sodium per vial , ie essentially “sodium-free”. However, depending on your body weight and your dose ofBeneFIX, you may receive several vials. This should be considered if you have been prescribed a low-salt diet.
3. How to use BeneFIX
Always use this medicine exactly as your doctor or pharmacist has told you. Ask your doctor or pharmacist if you are unsure.
Your doctor will decide on the dose of one of BeneFIX that you should take. The doseand duration of treatment depends on your individual need for factor IX replacement therapy and how quickly your body uses up factor IX, which will be checked regularly. You may notice a difference in the dose you receive if you switch from a plasma factor IX product to BeneFIX.
Your doctor may change the dose of BeneFIX you receive during treatment.
Preparation and administration
The instructions below are advice for the preparation and administration of BeneFIX. Follow your doctor’s instructions for venipuncture.
BeneFIX is given by intravenous (IV) infusion after reconstitution of the powder for solution for injection with the supplied diluent (sodium chloride (saline) solution) in the pre-filled syringe.
Always wash your hands before doing the following. Aseptic technique (meaning clean and germ-free) should be used during reconstitution .
Preparation :
BeneFIX is given by intravenous (IV) infusion after reconstitution with sterile diluent .
1. Allow the bottle of freeze-dried BeneFIX and the pre-filled syringe to reach room temperature.
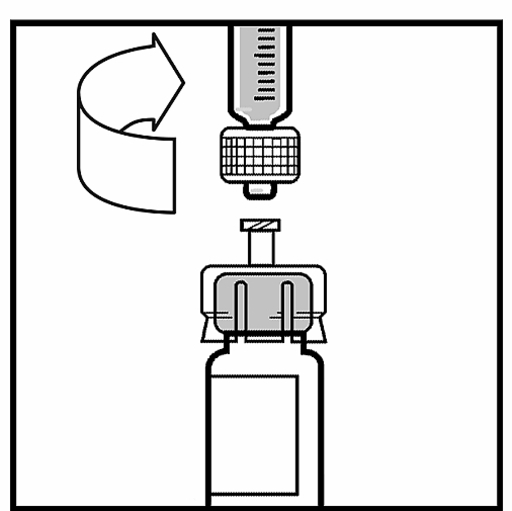
2. Remove the plastic cap from the BeneFIX bottle so that the middle part of the rubber stopper is visible.
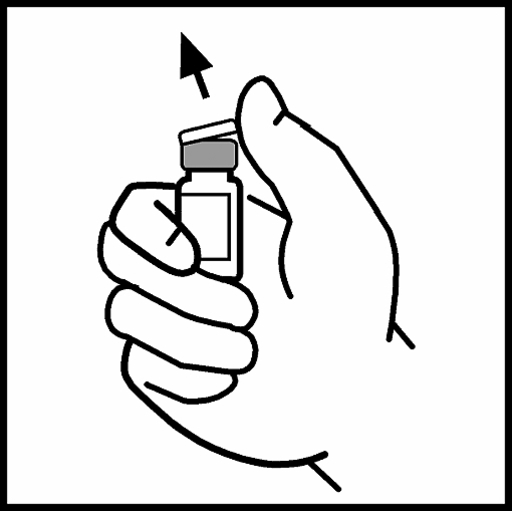
3. Clean the stopper with the supplied compress one with alcohol or use another antiseptic solution and let it dry. Do not touch the rubber stopper with your hand after cleaning and do not allow the stopper to come into contact with any surface.
4. Remove the cap from the bottle adapter’s clear plastic package. Do not remove the adapter from the package.
5. Place the bottle on a flat surface. While holding the adapter package, place the adapter over the bottle. Press firmly down on the package until the adapter clicks into place at the top of the bottle and the adapter needle passes through the rubber stopper of the bottle.
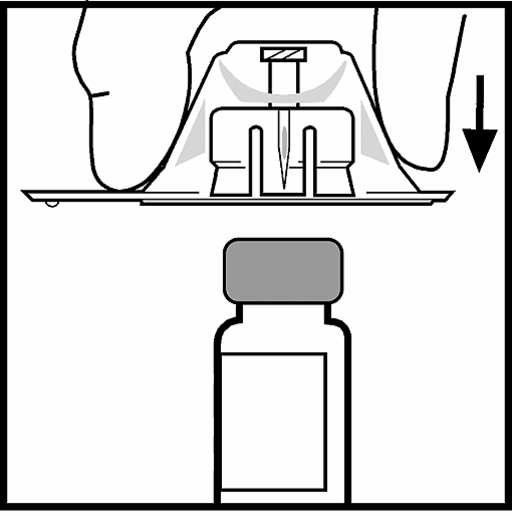
6. Lift the package off the adapter and discard the package.
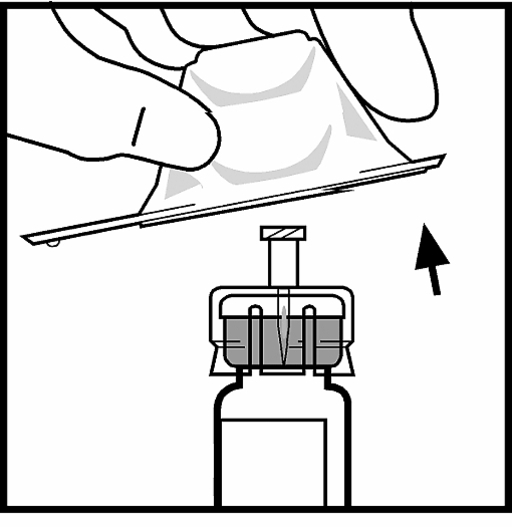
7. Attach the piston rod to the syringe with diluent by pressing and screwing firmly.
8. Break the plastic cap from the syringe with diluent by breaking the perforation on the cap. This is done by bending up and down until the perforation is broken. Do not touch the inside of the cap or the top of the syringe. The lid may need to be used again (unless the reconstituted BeneFIX is administered immediately), so set it aside by placing it on top.
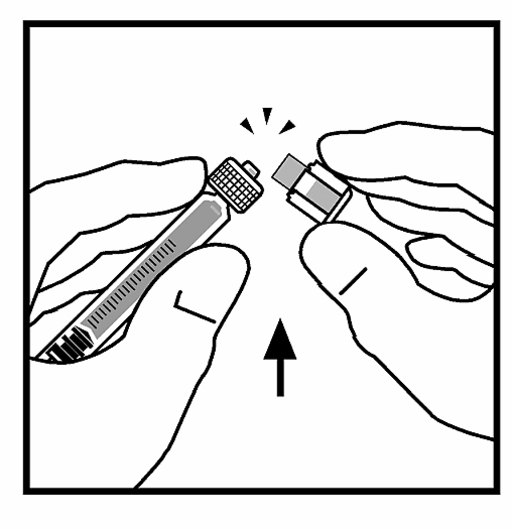
9. Place the bottle on a flat surface. Attach the diluent with the diluent to the bottle adapter by placing the top of the syringe in the adapter opening while pressing firmly and screwing the syringe clockwise until it has stabilized in place.
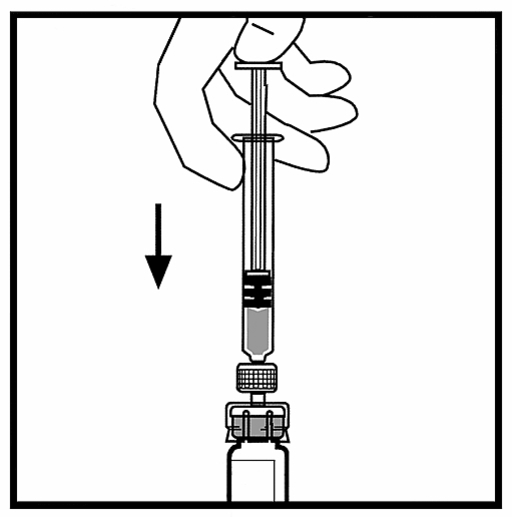
10. Slowly push in the plunger to transfer all the liquid to the bottle of BeneFIX.
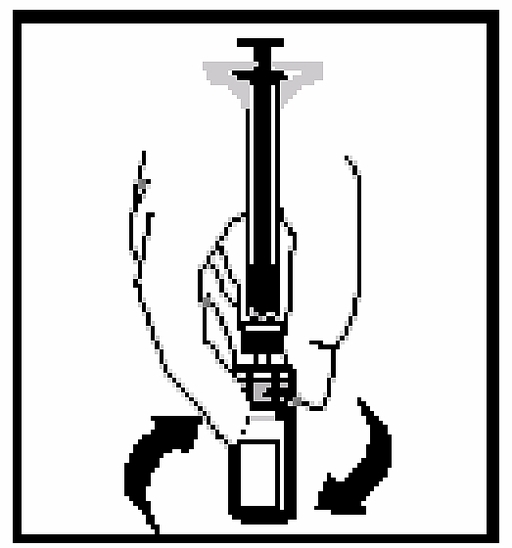
11. Without removing the syringe from the adapter, gently rotate the bottle until the powder has dissolved.
12. The finished solution should be inspected visually for particulate matter prior to administration . The solution should be clear and colorless.
Note: If you use more than one bottle of BeneFIX per injection , each bottle should be reconstituted according to the previous instructions. The diluent syringe should be removed, the bottle adapter left in place, and a separate large luer syringe (a device that connects the syringe to the bottle) can be used to draw the reconstituted contents from each bottle.
13. Make sure the syringe plunger is still fully depressed, and then turn the bottle upside down. Slowly withdraw all solution to the syringe.
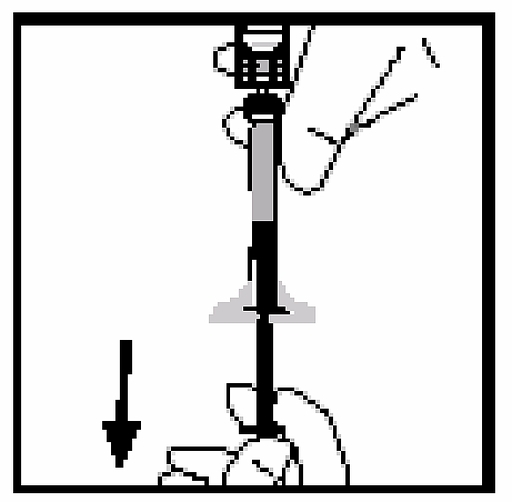
14. Remove the syringe from the bottle adapter by gently pulling and screwing the syringe counterclockwise. Discard the bottle with its fitted adapter.
Note: If the solution is not to be used immediately, carefully place the spray cap back in place. Do not touch the top of the syringe or the inside of the cap.
BeneFIX should be administered immediately or within 3 hours after reconstitution . The reconstituted solution should be stored at room temperature before administration .
Administration ( intravenous injection )
BeneFIX should be administered using the supplied pre-filled syringe or steriledisposable plastic lure syringe. In addition, the solution should be withdrawn from the vial using the vial adapter
BeneFIX should be injected intravenously over several minutes. Your doctor may change your recommended injection rate to make the injection more comfortable for you.
There have been reports of agglutination of red blood cells in the tubing / syringe during administration of BeneFIX. No side effects have been reported as a result of this observation. To reduce the risk of sticking, it is important to limit the amount of blood that enters the tube. Blood should not enter the syringe. If red blood cellsticking in the tubing / syringe is observed, discard all affected material (tubing, syringe and BeneFIX solution) and continue administration with a new pack.
As continuous infusion (drip) of BeneFIX has not been evaluated, BeneFIX must not be mixed with infusion solutions or given as a drip.
Any unused solution, empty vials, used needles and syringes should be disposed of in an appropriate manner to avoid injury to others.
If you use more BeneFIX than you should
Contact your doctor immediately if you have injected more BeneFIX than your doctor recommended.
If you stop using BeneFIX
Do not stop using BeneFIX without consulting a doctor.
If you have any further questions on the use of this product, ask your doctor, pharmacist or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Hypersensitivity reactions / allergic reactions
Allergic reactions such as hypersensitivity reactions are possible with BeneFIX. Such reactions may include swelling of the face or throat, burning and stinging sensation at the infusion site, chills, hot flashes, itching , headache, hives , low blood pressure , lethargy , nausea, restlessness, rapid heart rate, chest tightness, tingling, vomiting, wheezing. In some cases, these reactions have developed into severe anaphylaxis . Allergic reactions have occurred over time close to the development of factor IX inhibitors (see also “Warnings and Precautions”).
These reactions can be life-threatening. If allergic / anaphylactic reactions occur, discontinue infusion of BeneFIX immediately and contact your doctor or seek immediate emergency care. The treatment to be used depends on the nature and severity of the side effects (see also “Warnings and Precautions”).
Inhibitor development
Patients with haemophilia B may develop neutralizing antibodies (inhibitors) to factor IX. An indication that such inhibitors have been developed may be that an increase in the amount of BeneFIX normally required to treat a haemorrhage or continued haemorrhage after treatment is required. In such cases, it is recommended that a specialized haemophilia center be contacted. Your doctor may want to monitor you to see if any inhibitors develop (see “Warnings and Precautions”).
Renal impairment has been reported following high doses of plasma-derived factor IX products to induce immune tolerance in patients with haemophilia B and factor IX inhibitors and who have previously had allergic reactions (see also “Warnings and precautions”).
Blood clots
BeneFIX may increase the risk of thrombosis (formation of blood clots) in your body if you have risk factors for the formation of blood clots. A residual venous catheter can also be a risk factor for blood clots. There have been reports of severe thrombotic events, including life-threatening blood clots in severely ill newborns receiving BeneFIX by drip into a central venous catheter. Cases of peripheralthrombophlebitis (pain and redness of veins) and deep vein thrombosis (blood clots in the arms and legs) have also been reported. In most cases, BeneFIX had been administered by drip, which is not an approved method of administration.
Very common side effect are (can occur in more than 1 in 10)
- Headache
- Cough
- Fever
Common side effects are (may affect up to 1 in 10 people)
- Hypersensitivity reactions / allergic reactions
- Dizziness, altered taste
- Phlebitis (pain and redness of veins), hot flashes
- Vomiting, nausea
- Skin rash, hives
- Chest discomfort (including chest pain)
- Infusion site reactions (including itching and redness at the infusion site), pain and discomfort at the infusion site
Less common side effects are (may affect more than 1 in 100 people)
- Development of neutralizing antibodies (inhibitors)
- Cellulite at the infusion site (pain and redness of the skin)
- Fatigue, tremors
- Visual impairment (including blurred vision, blemishes and streaks of light in front of the eyes)
- Fast heart rate, low blood pressure
- Renal infarction (stop blood flow to the kidney)
Adverse reactions with no known frequency (frequency can not be calculated from the available data)
- Anaphylactic reaction
- Thrombotic events (abnormal blood clots)
- Lack of response to treatment (failure to stop or prevent bleeding episodes)
5. How to store BeneFIX
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and bottle label. The expiration date is the last day of the specified month.
BeneFIX must be stored at a maximum of 30 ° C and used before the expiry date which is stated on the label.
Do not freeze to avoid damaging the pre-filled syringe.
Use the reconstituted solution immediately, but not later than 3 hours after reconstitution .
Do not use BeneFIX if the solution is not clear and colorless.
Use only the pre-filled syringe that is included in the package for reconstitution . Other sterile disposable syringes may be used for administration .
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the packaging and other information
Content declaration
– The active substance is nonacog alfa ( recombinant coagulation factor IX). Each vial of BeneFIX nominally contains 250, 500, 1000, 1500, 2000 or 3000 IU nonacog alfa.
The other ingredients are sucrose, glycine , L-histidine, polysorbate 80. A diluent(0.234% sodium chloride solution) is also included in the preparation .
– After reconstitution with the supplied diluent (0.234% sodium chloride solution), each vial contains 50, 100, 200, 300, 400 or 600 IU / ml (see Table 1).
| Amount of BeneFIX per bottle | Amount of BeneFIX per 1 ml of reconstituted solution for injection |
|---|---|
| 250 IU | 50 IU |
| 500 IU | 100 IU |
| 1000 IU | 200 IU |
| 1500 IU | 300 IU |
| 2000 IU | 400 IU |
| 3000 IU | 600 IU |
What the medicine looks like and contents of the pack
BeneFIX is supplied as a powder for solution for injection in a glass vial and a diluent in a pre-filled syringe .
Package contents:
· A vial BeneFIX 250, 500, 1000, 1500, 2000 or 3000 IU powder
· A pre-filled syringe with diluent , 5 ml sterile 0.234% sodium chloride solution for injection for reconstitution , with flask
· A sterile bottle preparation adapter
· A sterile injection set
· Two compresses are with alcohol
· A patch
· A compress
Marketing Authorisation Holder
Pfizer Europe MA EEIG
Boulevard de la Plaine 17
1050 Brussels
Belgium
Manufacturer
Wyeth Farma SA
Autovia del Norte.A-1, Km. 23. Desvio Algete, Km. 1, 28700 San Sebastian de los Reyes, Madrid
Spain
Contact the representative of the marketing authorization holder to find out more about this medicine:
| Belgium / Belgique / BelgienPfizer SA / NVTel: +32 (0) 2 554 62 11 | LithuaniaPfizer Luxembourg SARL branches in LithuaniaTel. +3705 2514000 |
| BulgariaPfizer Luxembourg SAARЛ, Clone of BulgariaTel: +359 2 970 4333 | Luxembourg / LuxemburgPfizer SATel: +32 (0) 2 554 62 11 |
| Czech RepublicPfizer, spol. s roTel: +420 283 004 111 | HungaryPfizer Kft.Tel .: + 36 1 488 37 00 |
| DenmarkPfizer ApSTel: +45 44 20 11 00 | MaltaVivian Corporation Ltd.Tel: + 356 21 344610 |
| GermanyPfizer Pharma GmbHTel: +49 (0) 30 550055 51000 | The NetherlandsPfizer bvTel: +31 (0) 10 406 43 01 |
| EestiPfizer Luxembourg SARL Eesti subsidiaryTel: +372 666 7500 | NorwayPfizer ASTel: +47 67 52 61 00 |
| GreecePFIZER ΕΛΛΑΣ Α.Ε.:Ηλ: +30 210 67 85 800 | AustriaPfizer Corporation Austria Ges.mbHTel: +43 (0) 1 521 15-0 |
| SpainPfizer SLTel: +34 91 490 99 00 | PolandPfizer Polska Sp. z oo,Tel .: +48 22 335 61 00 |
| FrancePfizerTel: +33 (0) 1 58 07 34 40 | PortugalPfizer Laboratories, Lda.Tel: +351 21 423 5500 |
| CroatiaPfizer Croatia dooTel: + 385 1 3908 777 | RomaniaPfizer România SRLTel: +40 21 207 28 00 |
| IrelandPfizer Healthcare IrelandTel: 1800 633 363 (toll free)+44 (0) 1304 616161 | SloveniaPfizer Luxembourg SARLPfizer, a company in the field of pharmaceuticals, LjubljanaTel: + 386 (0) 1 52 11 400 |
| IcelandIcepharma hf.Phone: + 354 540 8000 | Slovak RepublicPfizer Luxembourg SARL, organizerTel: + 421-2-3355 5500 |
| ItalyPfizer SrlTel: +39 06 33 18 21 | Finland / FinlandPfizer OyPuh / Tel: +358 (0) 9 43 00 40 |
| ΚύπροςPFIZER ΕΛΛΑΣ Α.Ε. (CYPRUS BRANCH):Ηλ: +357 22 817690 | |
| LatviaPfizer Luxembourg SARL subsidiary LatvijāTel: +371 670 35 775 | United KingdomPfizer LimitedTel: +44 (0) 1304 616161 |