250 micrograms solution for injection in a pre-filled pen
chorionic gonadotropin alfa
What Ovitrelle is and what it is used for
What Ovitrelle is
Ovitrelle contains the drug “chorionic gonadotropin alfa” which is produced in a laboratory using a special recombinant DNA technology. Chorionic gonadotropin alfa is like a hormone found naturally in the body, called chorionic gonadotropin, which is involved in reproduction and fertility.
What Ovitrelle is used for
Ovitrelle is used with other medicines:
- To cause follicles (each containing an egg) to develop and mature in women undergoing assisted reproductive technology (a method that can help you conceive) such as ” in vitro fertilization”. Other drugs will be given first to make more follicles grow.
- To get the ovary to release an egg (ovulation) in women who can not produce eggs (“anovulation”) or women who produce too few eggs (“oligo-ovulation”). Other drugs will be given first for follicles to develop and mature.
What you need to know before you use Ovitrelle
Do not use Ovitrelle
- if you are allergic to chorionic gonadotropin alfa or any of the other ingredients of this medicine (listed in section 6).
- if you have a tumor in the hypothalamus or pituitary gland one (both are parts of the brain).
- if you have enlarged ovaries or fluid-filled blisters on the ovaries (ovarian cysts) of unknown origin.
- if you have unexplained vaginal bleeding.
- if you have cancer of the ovaries, uterus or breasts.
- if you have severe inflammation or blood clots in the veins (active thromboembolic diseases).
- if you have any other disorder that usually makes a normal pregnancy impossible, e.g. menopause or early menopause (ovarian failure) or malformations of the reproductive organs.
Do not use Ovitrelle if any of the above apply to you. If you are not sure, talk to your doctor before taking this medicine.
Warnings and cautions
Before starting treatment, your and your partner’s fertility should be examined by a doctor who has experience in treating fertility problems.
Ovarian overstimulation syndrome (OHSS)
This medicine may increase the risk of you developing OHSS. This means that the follicles develop too much and become large cysts.
If you experience lower abdominal pain, weight gain rapidly, nausea or vomiting, or difficulty breathing, do not inject yourself with Ovitrelle but talk to your doctor immediately (see section 4). If you develop OHSS, your doctor may tell you to abstain from sex or use a barrier contraceptive for at least four days.
The risk of OHSS is reduced if the recommended dose of Ovitrelle is used and if you are closely monitored throughout the treatment (eg measurement of estradiol levels in blood tests and ultrasound examination).
Multiple pregnancies and/or birth defects
When you use Ovitrelle, you are more likely to become pregnant with more than one child at a time (“multiple pregnancies”, usually twins) than if you were pregnant naturally. Multiple pregnancies can lead to medical complications for you and your children. When using assisted reproduction technology, the risk of multiple pregnancies is related to your age, the quality, and the number of fertilized eggs or embryos placed in the uterus. Multiple pregnancies and special characteristics of the couple with fertility problems (eg age) may also be associated with an increased risk of malformations.
The risk of multiple pregnancies decreases if you are carefully monitored throughout the treatment (eg measurement of estradiol levels in blood tests and ultrasound examination).
Ectopic pregnancy
Ectopic pregnancy (ectopic pregnancy) can occur in women with damaged fallopian tubes (the tubes that carry the egg from the ovary to the uterus). Therefore, your doctor should perform an early ultrasound examination to rule out the possibility of ectopic pregnancy.
Miscarriage
When using assisted reproductive technology or when the ovaries are stimulated to produce eggs, the risk of miscarriage is greater than for the average woman.
Blood clots (thromboembolic event)
Talk to your doctor before using Ovitrelle if you or a family member have ever had a blood clot in your leg or lung or a heart attack or stroke. You may be at greater risk of getting severe blood clots or existing blood clots may worsen with treatment with Ovitrelle.
A tumor is in the genitals
There have been reports of tumors in the ovaries and other genitals, both benign and malignant, in women who have undergone several treatments for infertility.
Pregnancy tests
You may get a false-positive test result if you take a pregnancy test on serum or urine for up to 10 days after taking Ovitrelle. Talk to your doctor if you are unsure.
Children and young people
Ovitrelle is not intended for use in children and adolescents.
Other medicines and Ovitrelle
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
Pregnancy and breastfeeding
Do not use Ovitrelle if you are pregnant or breastfeeding.
If you are pregnant or breastfeeding, talk to your doctor before using this medicine.
Driving and using machines
Ovitrelle is not expected to affect your ability to drive or use machines.
Ovitrelle contains sodium
This medicine contains less than 1 mmol (23 mg) sodium per dose, ie essentially ‘sodium-free’.
3. How to use Ovitrelle
Always use this medicine exactly as your doctor has told you. Ask your doctor or pharmacist if you are unsure.
How much to use
- The recommended dose is 1 pre-filled pen (250 micrograms / 0.5 ml) taken as an injection .
- Your doctor has explained exactly when to inject one.
How to use this medicine
- If you give yourself Ovitrelle, carefully read and follow the separate “Instructions for Use” in the box.
- Ovitrelle is for injection under the skin ( subcutaneously ).
- Each pre-filled pen is for single use only.
- Your doctor or nurse will show you how to use the pre-filled Ovitrelle pen to inject the medicine.
- Inject Ovitrelle exactly as your doctor or nurse has told you.
- After injection , discard the used needle safely and discard the pen.
If you use more Ovitrelle than you should
The effects of an overdose of Ovitrelle are unknown but ovarian hyperstimulation syndrome (OHSS) may occur. This is described in section 4.
If you forget to use Ovitrelle
If you forget to use Ovitrelle, talk to your doctor as soon as you find out.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Ovitrelle and see a doctor immediately if you notice any of the following serious side effects – you may need urgent medical attention:
- Allergic reactions such as rash, rapid or uneven pulse , swelling of the tongue and throat, sneezing, wheezing or severe breathing problems are very rare (may affect up to 1 in 10,000 people).
- Lower abdominal pain, distended abdomen or abdominal discomfort in combination with nausea or vomiting may be symptoms of ovarian hyperstimulation syndrome (OHSS). This may indicate that the ovaries are overreacting to the treatment and that large ovarian cysts have developed (see also section 2, under “Ovarian overstimulation syndrome (OHSS).” This is a common reaction (may affect up to 1 in 10 people).
- OHSS can become serious with clearly enlarged ovaries, decreased urine production, weight gain, difficulty breathing and possible fluid accumulation in the stomach or chest. This is less common (may affect up to 1 in 100 people).
- Severe thrombotic complications (thromboembolic events) sometimes independent of OHSS can occur in very rare cases. This may cause chest pain, shortness of breath, stroke or heart attack (see also section 2, under “Blood clot formation (thromboembolic event)”.
Other side effects are
Common (may affect up to 1 in 10 people)
- Headache.
- Local reactions at the injection site, such as pain, redness or swelling.
Uncommon (may affect up to 1 in 100 people)
- Diarrhea.
How to store Ovitrelle
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiration date is the last day of the specified month.
Store in a refrigerator (2 ° C-8 ° C). Do not freeze.
Do not use Ovitrelle if you see visible signs of deterioration if the solution contains particles or is not clear.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Contents of the package and other information
Content declaration
- The active substance is chorionic gonadotropin alfa produced by recombinant DNA technology.
- Each pre-filled pen contains 250 micrograms of chorionic gonadotropin alfa in 0.5 ml (equivalent to approximately 6,500 international units, IU).
- The other ingredients are mannitol, methionine, disodium phosphate dihydrate, sodium dihydrogen phosphate monohydrate, poloxamer 188, phosphoric acid (for pH adjustment), sodium hydroxide (for pH adjustment) and water for injections.
What the medicine looks like and contents of the pack
- Ovitrelle is supplied as a clear, colorless to yellowish solution for injection in a pre-filled pen .
- Each pen contains 0.5 ml of solution.
- The product is supplied in packs of 1 pre-filled pen and 2 injection needles (one extra).
Marketing Authorisation Holder
Merck Europe BV, Gustav Mahlerplein 102, 1082 MA Amsterdam, The Netherlands
Manufacturer
Merck Serono SpA, Via delle Magnolie 15, 70026 Modugno (Bari), Italy
This leaflet was last modified 10/on 2021. Further information on this medicine is available on the European Medicines Agency website: http://www.ema.europa.eu.
Instructions for use
OVITRELLE 250 micrograms solution for injection in a pre-filled pen
chorionic gonadotropin alpha
CONTENT
- How to use Ovitrelle pre-filled pen
- Before you use Ovitrelle pre-filled pen
- Prepare Ovitrelle pre-filled pen for injection
- Setting dose one – “How to set dose one to 250”
- Inject dos en
- After injection one
Warning! Read this manual before using the Ovitrelle pre-filled pen. Follow the instructions exactly as they may differ from your previous experience.
1. How to use the Ovitrelle pre-filled pen
- The pen is for subcutaneous injection only .
- Inject Ovitrelle exactly as your doctor or nurse has told you.
- This pen is for single use only. Do not share the pen with anyone else.
Before you use Ovitrelle pre-filled pen
| Wash your hands with soap and water. | |
Find a clean and flat surface. | |
| Check the expiration date on the pen label. | |
| Gather everything you need and post it: |
| 1. dose setting button2. dosdisplay3rd piston4. container5. threaded needle connection6. protection for pen | 7. peelable seal8. removable needle9. inner needle guard10. outer needle guard11. alcohol swabs12. containers for sharp objects |
Note! Alcohol wipes and containers for sharp objects are not included in the packaging.
3. Prepare Ovitrelle pre-filled pen for injection
3.1 Remove the cover on the pen
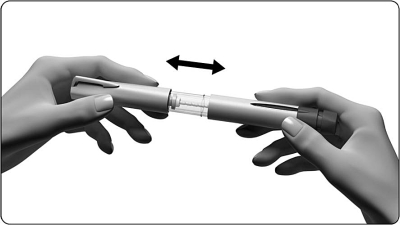
3.2 Prepare the needle for injection
| Warning: If the pull-off seal is damaged or loose, do not use the needle. Throw it in a container for sharp objects. Ask your doctor or pharmacist how to get a new needle. |
3.3 Connect the needle
3.4 Look closely at the tip of the needle for a small drop/drops of liquid
| Warning: If you do not see a small drop/drop at or near the tip of the needle, follow the steps on the next page. |
If you do not see a small drop/drops at or near the tip of the needle:
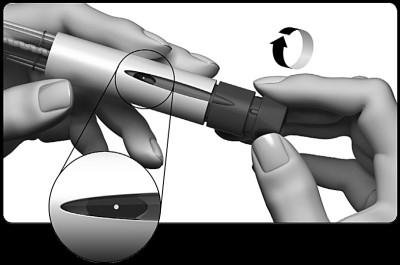
- Carefully turn the dose setting knob clockwise until you see a dot (●) on the dose display. If you pass this mode, simply turn the dose setting knob back to the point (●).
- Hold the pen with the needle pointing upwards.
- Gently tap the container.
- Press the dose setting button as far as it will go . A small drop of liquid is visible on the tip of the needle. This indicates that your pre-filled pen is ready for injection .
- If you do not see any liquid, you can try a second time (you can do this no more than twice) by starting from step 1 in the section “If you do not see a small drop / drops at or near the tip of the needle” above.
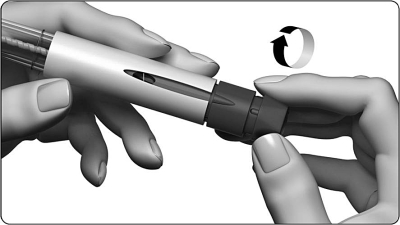
4. Set dose one to 250
- Carefully turn the dose setting knob clockwise. The dose display shows a straight line and you must continue to turn until you can read the number ” 250 “.
- Do not press or pull the dose setting knob when turning it.
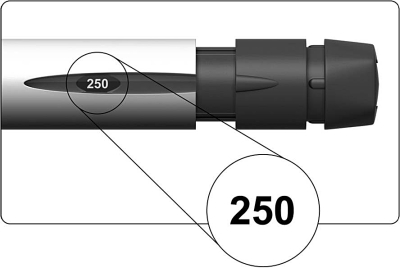
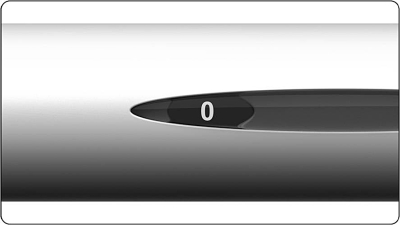
- The dose display should show “250” as shown in the figure below.
5. Inject dos en
5.1 Choose an injection site in the area where your doctor or nurse has told you to inject one.
5.2 Clean the skin at the injection site by wiping it with an alcohol swab.
5.3 Check again that the number on the dose display is “250”. If it does not show this, you must adjust it (see the step “4. Set dose one to 250”).
5.4 Inject a dose that your doctor or nurse has taught you
6. After injection a
6.1 Check that the dose display shows 0.
- This confirms that the entire dose has been injected. Do not try to inject a second time.
- If the dose display does not show 0, contact your doctor or pharmacist.
6.2 Remove the needle after injection
- Hold the pre-filled pen firmly in the container.
- Carefully attach the outer needle cover to the needle.
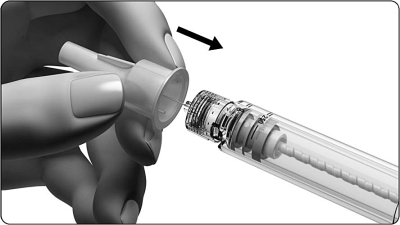
- Grasp the outer needle cover and unscrew the needle.
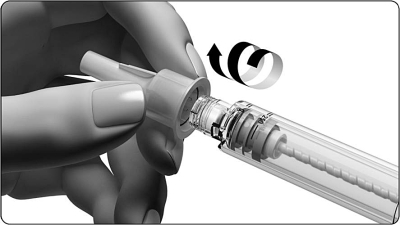
- Be careful not to stick it on the needle.
- Now put the cover back on the pen.
6.3 Disposal
| Use the needle and pen only once. When you have finished your injection, discard the used needle safely. Discard the pen. It is best to put it back in its original packaging. When the pen is empty, ask your pharmacist how to dispose of it. |