1 mg/ml nasal spray, solution
What Otrivin Menthol (without preservatives) is and what it is used for
Otrivin Menthol is used for the short-term treatment of nasal congestion in colds and acute sinusitis.
Otrivin Menthol is a nasal spray with a decongestant effect.
What you need to know before using Otrivin Menthol (without preservatives)
Do not use Otrivin Menthol (without preservatives)
- if you are allergic to xylometazoline or any of the other ingredients of this medicine (listed in section 6).
- if you have had a pituitary gland operated on or after surgery where the meninges may have been damaged
- if you have glaucoma with a narrow ventricular angle (increased pressure in the eyes)
- if you have chronic rhinitis with very dry nasal passages ( rhinitis sicca or atrophic rhinitis ).
Tell your doctor or pharmacist if any of the above apply to you. Under these circumstances, Otrivin Menthol is not suitable for you.
Warnings and cautions
Talk to your doctor or pharmacist before using Otrivin Menthol if:
- you have high blood pressure
- you have cardiovascular disease (eg long QT syndrome)
- you have an overactive thyroid gland ( hyperthyroidism
- you have diabetes
- you have prostate enlargement ( prostate hypertrophy )
- you have a tumor in the adrenal gland that produces large amounts of adrenaline and norepinephrine ( pheochromocytoma )
- you are being treated with an antidepressant drug called a monoamine oxidase inhibitor ( MAOI ) or if you have been using it for the last 2 weeks
Do not use Otrivin Menthol without talking to your doctor or pharmacist if any of the above apply to you.
People who are sensitive to drugs with an adrenaline-like effect (eg adrenaline, ephedrine ) may experience side effects in the form of sleep disturbance, dizziness, tremors, palpitations, or high blood pressure and should therefore not use Otrivin Menthol without first talking to a doctor.
To avoid drug-induced nasal congestion, which can lead to physical drug dependence, Otrivin Menthol should not be used for more than 10 days.
Otrivin Menthol should not be used in the eyes or mouth. Do not exceed the recommended dose, especially in children and the elderly.
Children and young people
Otrivin Menthol should not be used by children under 12 years of age.
Other medicines and Otrivin Menthol (without preservatives)
Treatment with Otrivin Menthol may be affected if this medicine is taken at the same time as tricyclic (eg clomipramine, amitriptyline) or tetracyclic (eg protein) antidepressants. Treatment with Otrivin Menthol may affect the effect of monoamine oxidase inhibitors. This applies to concomitant use and when monoamine oxidase inhibitors have been used during the last two weeks before treatment with Otrivin Menthol.
Pregnancy, breastfeeding and fertility
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor for advice before using this medicine. As a precautionary measure, Otrivin Menthol should not be used by pregnant or breastfeeding women unless directed to do so by a doctor.
Driving and using machines
Otrivin Menthol does not affect your ability to drive or use machines.
How to use Otrivin Menthol (without preservatives)
Always use this medicine exactly as your doctor or pharmacist has told you. . Ask your doctor or pharmacist if you are unsure.
Adults and children over 12 years :
1 spray shower in each nostril if necessary 2-3 times daily. Do not use more than 3 spray showers daily in each nostril. Used for a maximum of 10 consecutive days. Another dose according to the doctor’s prescription. Each spray of Otrivin Menthol contains 0.14 ml of solution.
It is recommended that the last dose of the day be taken just before bedtime. Other dosing takes place according to a doctor’s prescription.
When used for more than 10 consecutive days, Otrivin Menthol may cause nasal congestion.
Instructions for use
1. Sniff out the nose. Remove the clear plastic cap.
2. Do not cut off the tip. The spray bottle is ready to be used.
Before first use, prepare the pump by spraying 4 times. When the pump is prepared, it stays charged throughout the treatment period. If the spray does not deliver during pumping or if it has not been used for more than 6 days, the pump needs to be prepared again with 4 sprays as before the first use. Be very careful not to spray on your eyes or mouth.
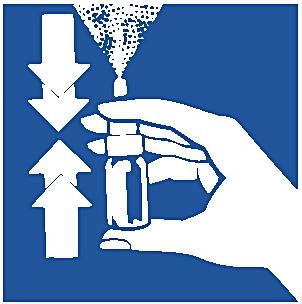
4. Hold the spray bottle as shown.
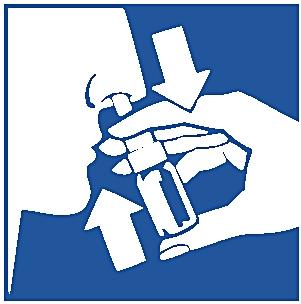
5. Carefully lean forward and insert the tip into one nostril.
6. Spray while inhaling gently through the nose.
7. Repeat the procedure in the other nostril.
To avoid spreading the common cold, the nasal spray should only be used by one person.
If you use more Otrivin Menthol (without preservatives)
If you have ingested too much medicine or if e.g. If a child has ingested the medicine by mistake, contact a doctor or hospital for risk assessment and advice.
If you forget to use Otrivin Menthol (without preservatives)
Do not take a double dose to make up for a forgotten dose.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Stop using Otrivin Menthol and seek medical attention immediately if you experience any of the following reactions that may be signs of an allergic reaction:
• difficulty breathing or swallowing
• swelling of the face, lips, tongue, or throat
• severe itching of the skin with a red rash or lumpy skin
Common (may affect up to 1 in 10 people): Headache, nausea, burning or local irritation of the nose, dry nasal mucosa
Uncommon (may affect up to 1 in 100 people): Nasal bleeding
Has been reported (occurs in an unknown number of users): Allergic reactions (rash, itching ), irregular or rapid heartbeat, increased blood pressure, and transient blurred vision.
If Otrivin Menthol is used for more than 10 consecutive days, nasal congestion may occur.
If any of the side effects gets serious, or if you notice any side effects not listed in this leaflet, please tell your doctor or pharmacist.
How to store Otrivin Menthol (without preservatives)
Keep out of sight and reach of children.
Do not store above 25 ° C.
Do not use this medicine after the expiry date which is stated on the label and carton after EXP. The expiration date is the last day of the specified month.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Contents of the package and other information
Content declaration
- The active substance is xylometazoline hydrochloride 1.0 mg / ml.
- The other ingredients are menthol, cineole, disodium edetate, sodium dihydrogen phosphate dihydrate, disodium phosphate dodecahydrate, sodium chloride, sorbitol, macrogolglycerol hydroxystearate, purified water.
The contents are free of preservatives.
What the medicine looks like and contents of the pack
10 ml HD polyethylene bottle with dosing spray pump.
Marketing Authorization Holder and Manufacturer
Marketing Authorisation Holder
GlaxoSmithKline Consumer Healthcare A / SApS, PO Box 61, 2610 Rødovre, Denmark
Manufacturer
GlaxoSmithKline Consumer Healthcare ApS,
Delta Park 37
2665 Vallensbæk Strand, Denmark