eye drops, solution, single-dose container
Carmelo’s sodium
1. What Cellufluid is and what it is used for
Cellufluid is a tear substitute and contains a lubricant called carmellose sodium. Cellufluid is used to relieve symptoms due to dry eyes (eg chafing, burning sensation, irritation, and dryness) caused by not producing enough tear fluid to keep the eye moist.
Carmellose sodium contained in Cellufluid may also be approved for the treatment of other conditions not mentioned in this product information. Ask your doctor, pharmacist, or other healthcare professional if you have any further questions and always follow their instructions.
2. What you need to know before using Cellufluid
Do not use Cellufluid
- if you are allergic (hypersensitive) to caramel sodium or to any of the other ingredients of this medicine (listed in section 6).
Warnings and cautions
- If irritation, pain, redness, and visual disturbance occur, or if the symptoms worsen, stop using this medicine and contact your doctor or pharmacist.
Other medicines and Cellufluid
Tell your doctor or pharmacist if you are taking or have recently taken or might take any other medicines, including medicines obtained without a prescription.
If you use other eye drops, wait at least 15 minutes between taking the other drops and Cellufluid.
Pregnancy and breastfeeding
Cellulose can be used during pregnancy and lactation.
Driving and using machines
Transient blurred vision may occur. If you experience transient blurred vision, do not drive or use machines until vision is clear.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased vigilance. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. Descriptions of these effects and side effects can be found in other sections.
Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
3. How to use Cellufluid
Always use this medicine exactly as described in this leaflet or as your doctor, pharmacist or nurse have told you. If you are not sure, talk to your doctor, pharmacist, or nurse.
The recommended dose is 1-2 drops of Cellufluid in the affected eye / each affected eye, 4 times daily or as needed.
You do not need to remove your contact lenses before using Cellufluid.
Make sure that pipette one is unbroken before using it. The solution should be used immediately after opening pipette one. To avoid contamination or possible eye damage, do not allow the pipette opening to come into contact with the eye or anything else. Wash hands before use.
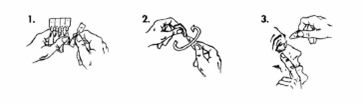
- Remove a pipette from the strip.
- Hold the pipette upright (wing at the top) and turn off the wing.
- Carefully pull the lower eyelid down to form a pocket. Invert the pipette upside down and squeeze it to push a drop into the eye (s) that needs to be treated. Blink your eyes a few times.
Do not reuse pipette one even if there is a solution left in it. It is very important that you throw away the solution and do not keep it.
If you use more Cellufluid than you should
this does not involve any risks. If you are worried, talk to your doctor or pharmacist.
If you forget to use Cellufluid
Drop a single drop into the eye that needs to be treated as soon as you remember, then continue dosing one according to your normal routines. Do not take a double dose to make up for a forgotten dose.
If you have any further questions on the use of this product, ask your doctor, pharmacist, or nurse.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
The side effects are presented in the following categories, depending on how often they occur:
| Very common | May affect more than 1 user in 10 |
|---|---|
| Usual | May affect up to 1 in 10 users |
| Less common | May affect up to 1 in 100 users |
| Rare | May affect up to 1 in 1,000 users |
| Very rare | May affect up to 1 in 10,000 users |
| No known frequency | The frequency cannot be calculated from the available data |
The following side effects may occur when using Cellufluid:
| Usual | Eye irritation (including burning and discomfort), eye pain, itchy eyes, visual disturbances. |
|---|---|
| No known frequency | Allergic reactions (including eye allergy), blurred vision, sticky eyes, watery eyes, red eyes, eye damage to the surface of the eye due to the tip of the pipette touching the eye during use. |
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Medical Products Agency, www.lakemedelsverket.se. By reporting side effects, you can help increase drug safety information.
5. How to store Cellufluid
Keep this medicine out of the sight and reach of children.
Do not store above 25 ° C.
Do not use this medicine after the expiry date which is stated on the pipette one and the carton after EXP: The expiration date is the last day of the specified month.
Do not use this medicine if the Cellufluid package does not appear to be unopened. Do not use Cellufluid if the solution changes color or becomes cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the packaging and other information
Content declaration
- The active substance is carmellose sodium 5 mg / ml.
- The other ingredients are sodium chloride, sodium lactate, potassium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, and purified water. Small amounts of sodium hydroxide or hydrochloric acid may have been added to correct the pH of the solution (a measure of the acidity of the solution).
The ingredients in Cellufluid are chosen to mimic your natural composition of tears.
What the medicine looks like and the contents of the pack
Cellufluid is a clear, colorless to pale yellow eye drop solution in small transparent pipettes (similar to a bubble, so-called single-dose containers ). The pipettes have a breakable closure in the form of a rotatable wing. Each pipette contains 0.4 ml of eye drop solution.
Each pack contains 5, 30, or 90 single-dose containers. Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Allergan Pharmaceuticals Ireland
Castlebar Road
Westport
County Mayo
Ireland.