Film-coated tabletsEstradiol Noretisterone
1. What Activelle is and what it is used for
Activelle® is the preparation that is used for continuous hormonal substitution therapy ( Hormone Replacement Therapy, HRT ). It contains two female sex hormones; estrogen and progestogen. Activelle is used for women whose period has stopped ( menopause ), at least 1 year after their last natural menstrual period
Activelle is used to:
Relieve symptoms during and after menopause
When menstruation ceases ( menopause ), a woman’s estrogen drops. It can cause problems such as sweating and hot flashes. Activelle relieves these symptoms after menopause. Activelle should only be used if the problems cause problems in daily life.
Prevent osteoporosis
After menopause, some women suffer from osteoporosis. Discuss all possible options with your doctor.
If you have an increased risk of fractures (bone fractures) and other medicines that are not suitable for you, you can use Activelle to prevent osteoporosis after menopause.
Activelle is prescribed to women who have their uterus left and who had their last period more than a year ago.
Experience in the treatment of women over 65 years of age with Activelle is limited.
2. What you need to know before taking Activelle
Medical background and regular check-ups
The use of HRT involves risks that must be taken into account when deciding to start treatment or continue an ongoing treatment.
Experience is limited for the treatment of women whose menstruation has stopped prematurely (when the ovaries have stopped working or the uterus has been removed). If you belong to that group, the risks of HRT may be different. Talk to your doctor.
Before starting treatment (or resuming treatment), your doctor will ask about your own, and your family’s, medical background. Your doctor may do a general medical and gynecological examination, which also includes an examination of your breasts.
Once you have started treatment with Activelle, you should have regular medical check-ups, at least once a year. During these check-ups, you should discuss with your doctor the benefits and risks of continued treatment.
Perform regular breast examinations according to your doctor’s recommendations.
Do not take Activelle
if any of the following apply to you. If you are not sure, talk to your doctor before taking Activelle.
Do not take Activelle:
- if you have or have had breast cancer or there is a suspicion that you may have it.
- if you have or have had estrogen-dependent cancer, such as cancer of the uterine lining (endometrium) or if there is a suspicion of such cancer.
- if you have unexpected genital bleeding that has not been investigated by a doctor.
- if you have severe thickening of the uterine lining ( endometrial hyperplasia ) and are not treated for it.
- if you have or have had a blood clot in a vein ( thrombosis ) in your legs (deep vein thrombosis ) or in your lungs ( pulmonary embolism ).
- if you have a coagulation disorder, a condition with an increased risk of blood clots (lack of protein C, protein S, or antithrombin).
- if you have or have had a disease caused by blood clots in the arteries are as heart attack, stroke (stroke), or angina.
- if you have or have had any liver disease and still have abnormal liver values.
- if you have porphyria, a rare inherited blood disorder.
- if you are allergic (hypersensitive) to estradiol, norethisterone acetate, or any of the other ingredients of Activelle (see section 6 “Contents of the pack and other information”).
If any of the above happens to you the first time you use Activelle, stop taking Activelle, and contact your doctor immediately.
Warnings and cautions
Talk to your doctor if you have or have had any of the following problems before starting treatment. They may recur or worsen during treatment with Activelle. If this happens, check with your doctor more often:
- If you have any disease affecting the lining of the uterus, as well as muscle knots ( fibroids ), endometriosis, or have had endometrial hyperplasia (severe thickening of the uterine lining).
- If you have an increased risk of getting a blood clot (see below “Blood clots in a vein ( thrombosis )”)
- If a close relative has had breast cancer or other estrogen-dependent cancer.
- High blood pressure
- Liver disease eg liver adenoma (benign tumor )
- Diabetes
- Gallstone disease
- If you get a migraine or severe headache
- If you have systemic lupus erythematosus, ( SLE ) – an autoimmune disease that affects many organs in the body
- Epilepsy
- Asthma
- Otosclerosis (ossification of the middle ear leading to a hearing loss)
- Hypertriglyceridemia (elevated blood lipids)
- Fluid retention due to heart or kidney disease
- Lactose intolerance.
You should contact a doctor immediately and discontinue treatment with Activelle
if any of the following occurs:
- Some of what is mentioned in the section “Do not take Activelle”
- If skin or whites of the eyes turn yellow (jaundice); it may be a symptom of liver disease
- If your blood pressure rises sharply (symptoms may include headache, fatigue, or dizziness)
- If you are experiencing migraine-like headaches for the first time
- If you become pregnant
- If you get symptoms of a blood clot, such as:
– painful swelling and redness of the legs
– sudden chest pain
– difficulty breathing.
For more information, see below “Blood clots in a vein ( thrombosis )”.
Note: Activelle is not a contraceptive. If it is less than 12 months since your last period, or if you are under 50, you may still need to use contraception to avoid pregnancy. Consult your doctor.
HRT and cancer
Severe thickening of the uterine lining ( endometrial hyperplasia ) and cancer of the uterine lining (endometrial cancer)
Use of HRT with estrogen alone increases the risk of severe thickening of the uterine lining and cancer of the uterine lining.
The progestogen in Activelle protects you against this extra risk.
Irregular bleeding
Irregular spotting or spotting may occur during the first 3-6 months of taking Activelle.
But about the bleeding:
- lasts longer than 6 months
- starts after taking Activelle for 6 months
- continues after you stop taking Activelle
you should see a doctor as soon as possible.
Breast cancer
Data suggest that treatment with combined estrogen-progestogen and possibly also with estrogen alone increases the risk of breast cancer. The extra risk depends on how long you take HRT. The increased risk is seen within a few years but returns to normal within a few years (maximum 5 years) after treatment has stopped.
Comparison
For women aged 50–79, who do not take HRT, an average of 9–17 out of 1,000 will be diagnosed with breast cancer over a 5-year period.
For women aged 50–79 who take estrogen – the progestogen HRT for 5 years, 13–23 out of 1,000 users will be diagnosed (ie 4–6 extra cases).
Check your breasts regularly. Contact a doctor if you notice changes such as:
- indentations or pits
- changes of the nipple
- nodules you can see or feel.
It is also recommended that you participate in a mammography examination when you are called to do so. At the mammogram, it is important that you tell the nurse/healthcare professional who performs the examination that you are using HRT, as this medicine may increase the density of the breasts. An increased density in the breasts can make it more difficult to detect lumps on the mammography images.
Ovarian cancer ( ovarian cancer )
Ovarian cancer is rare – much more rare than breast cancer. Use of HRT with estrogen alone or combined estrogen – progestogens have been associated with a slightly increased risk of ovarian cancer.
The risk of ovarian cancer varies with age. The diagnosis of ovarian cancer will, for example, be made on about 2 women out of 2000 aged 50 to 54 who do not take HRT for a 5-year period. For women who have taken HRT for 5 years, there will be about 3 cases per 2000 users (ie about 1 extra case).
How HRT affects the heart and blood circulation
Blood clots in a vein ( thrombosis )
The risk of blood clots in the veins is 1.3–3 times higher for women who take HRT than for those who do not, especially during the first year of treatment.
Blood clots can be serious. If a blood clot ends up in the lungs, it can cause chest pain, shortness of breath, collapse, or even lead to death.
You are more likely to get a blood clot in a vein if any of the following apply to you. Tell your doctor if any of the following apply to you:
- You have not been able to walk or stand for a long time due to a major operation, injury or illness (see also section 3, “If you need surgery”)
- You are severely overweight ( BMI over 30 kg / m 2 )
- You have a coagulation disorder that requires long-term treatment with drugs that prevent blood clots
- If a close relative has had a blood clot in the bone, lung, or another organ
- You have SLE (systemic lupus erythematosus)
- You have cancer.
The symptoms of blood clots are described in the section “You should contact a doctor immediately and discontinue treatment with Activelle”.
Comparison
For women in their 50s who do not take HRT, an average of 4-7 out of 1,000 people are expected to have a blood clot in a vein over a 5-year period.
For women in their 50s who have taken HRT with estrogen-progestogen for more than 5 years, 9-12 out of 1,000 users are expected to get a blood clot in a vein (ie 5 extra cases).
Heart disease (heart attack)
There is no evidence that HRT prevents heart attacks.
For women over the age of 60 who take HRT with estrogen-progestin, the risk of developing heart disease is slightly higher than those who do not take HRT.
Stroke (apoplexy)
The risk of stroke is about 1.5 times higher for those who take HRT compared to those who do not. The risk of stroke is age-dependent, therefore the number of cases of stroke increases due to the use of HRT with increasing age.
Comparison
For women in their 50s who do not take HRT, an average of 8 out of 1,000 people is expected to have a stroke over a 5-year period.
For women in their 50s who take HRT for more than 5 years, 11 out of 1,000 users are expected to have a stroke (ie 3 extra cases).
Other conditions
The use of HRT preparations does not prevent memory loss. The risk of memory loss may be slightly higher in women who start using HRT after the age of 65. Consult your doctor.
Other medicines and Activelle
Some medicines may affect the effect of Activelle, which may lead to irregular bleeding. The following applies:
- Drugs for epilepsy (eg phenobarbital, phenytoin, and carbamazepine)
- Medicines for tuberculosis (eg rifampicin and rifabutin)
- Drugs for HIV – infection (for example, nevirapine, efavirenz, ritonavir, and nelfinavir)
- An herbal medicine containing St. John’s wort ( Hypericum perforatum )
- Medicines for hepatitis C infection (such as telaprevir).
Other medicines may increase the effect of Activelle:
- Medicines containing ketoconazole (an antifungal agent).
Activelle may affect cyclosporine when used concomitantly.
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription and herbal remedies.
Results from blood test analyze
If you need to take a blood sample, tell your doctor or the person taking the blood sample that you are taking Activelle as it may affect the results of some tests.
Activelle with food and drink
The tablets can be taken with or without food and drink.
Pregnancy and breastfeeding
Pregnancy: Activelle is for women whose period has stopped. If you become pregnant, stop taking Activelle and consult a doctor.
Breast-feeding: You should not use Activelle if you are breast-feeding.
Driving and using machines
Activelle has no known influence on the ability to drive or use machines.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires sharpened attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. Descriptions of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
Important information about an ingredient in Activelle
Activelle contains lactose monohydrate. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking Activelle.
How to take Activelle
Always take this medicine exactly as your doctor has told you. Ask your doctor or pharmacist if you are unsure.
Take one tablet daily at about the same time. When you have used up all 28 tablets in calendar pack one, you continue immediately without interruption with the next pack.
For further information on how to use calendar packaging, see “Instructions for use” at the end of the leaflet.
You can start treatment with Activelle on any day. However, if you switch from an HRT preparation when you have your monthly bleeding, you should start treatment immediately after the bleeding has stopped.
Your doctor will strive to give you the lowest dose, which will give you relief of symptoms, and that you should use Activelle for the shortest possible time. Talk to your doctor if you do not get any relief from the symptoms, or feel that the dose is too high.
If you forget to take Activelle
If you forget to take your tablet, you should take it within 12 hours from the usual time. If more than 12 hours have passed, skip this dose and take the next tablet, as usual, the next day. Do not take a double dose to make up for a forgotten tablet. Missing a dose can increase the likelihood of breakthrough bleeding and spotting if you still have your uterus.
If you stop taking Activelle
If you want to stop taking Activelle, you should first discuss this with your doctor. The doctor will explain what it means to stop taking the tablets and inform you about other alternatives.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
If you need surgery
If you are going to have an operation, tell your surgeon that you are taking Activelle. You may need to stop taking Activelle for 4 to 6 weeks before surgery to avoid the risk of blood clots (see section 2, “Blood clots in a vein ( thrombosis )”). Ask your doctor when it is appropriate to start taking Activelle again.
4. Possible side effects
Like all medicines, Activelle can cause side effects, although not everybody gets them.
The following diseases are more common in women who take HRT than those who do not:
- Breast cancer
- Severe thickening of the uterine lining ( endometrial hyperplasia ) or cancer of the uterine lining (endometrial cancer)
- Ovarian cancer ( ovarian cancer )
- Blood clots in veins in bones or lungs (venous thromboembolism )
- Heart disease
- Stroke (apoplexy)
- Probable memory loss, if treatment with HRT is started after the age of 65.
For more information on these side effects, see section 2 “What you need to know before taking Activelle”.
Hypersensitivity / allergy (uncommon side effect – affects 1 to 10 users in 1,000)
Although less common, hypersensitivity/allergy may occur and include one or more of the following symptoms: hives, itching, swelling, difficulty breathing, low blood pressure (pale cold skin, palpitations), dizziness, sweating. These may be signs of a so-called anaphylactic reaction/shock. If you get any of the listed symptoms, stop taking Activelle immediately, and contact a doctor immediately.
A very common side effect is (occurring in more than 1 in 10)
- Chest pain or tenderness in the breasts
- Vaginal bleeding.
Common adverse s (affecting 1-10 in 100)
- Headache
- Weight gain due to accumulation of fluid in the body
- Inflammation of the vagina
- Migraine or worsening of current migraine
- Fungal infection of the vagina
- Depression or worsening of current depression
- Nausea
- Breast enlargement or tightness in the breasts (breast edema)
- Back pain
- Uterine fibroid (benign tumor ), worsening or recurrence of the tumor
- Swollen arms and legs (peripheral edema )
- Weight gain.
Less common side effects are (affecting 1-10 of 1 000)
- Roughness, abdominal pain, tension, the feeling of discomfort in the stomach or flatulence
- Acne
- Hair loss ( alopecia )
- Abnormal (male) hair growth
- Itching or hives ( urticaria )
- Superficial venous inflammation associated with a blood clot
- Leg cramps
- No effect
- Allergic reaction
- Nervousness.
Rare side effects are (affecting 1-10 10 000)
- Blood clots in the blood vessels of the legs or lungs (deep vein thrombosis, pulmonary embolism ).
Rare side effects are (by less than 1 in 10 000)
- Uterine cancer (endometrial cancer)
- Severe thickening of the uterine lining ( endometrial hyperplasia )
- Increased blood pressure or worsening of high blood pressure
- Gallbladder disease, gallstones, deterioration, or new occurrence of gallstones
- Strong secretion of sebum, skin rash
- Acute or recurrent edema ( angioneurotic edema )
- Insomnia, dizziness, anxiety
- Altered sex drive
- Visual disturbances
- Weight loss
- Vomiting
- Heartburn
- Itching in the abdomen
- Myocardial infarction and stroke.
Other side effects are HRT with estrogen-progestogen
- The disease of the gallbladder
- Various skin diseases:
– dark skin spots, especially on the face and neck, so-called “pregnancy spots” (chloasma)
– painful reddish-purple bumps on the skin (erythema nodosum)
– erythema multiforme
– red or purple discolorations on the skin and/or mucous membranes ( vascular purpura)
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Medical Products Agency, www.lakemedelsverket.se. By reporting side effects, you can help increase drug safety information.
5. How to store Activelle
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label and the carton after “EXP”. The expiration date is the last day of the specified month
Do not store above 25 ° C.
Store in a cold place.
Store in the outer carton. Sensitive to light.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the packaging and other information
Content declaration
– The active substances are estradiol 1 mg (as estradiol hemihydrate) and norethisterone acetate 0.5 mg.
The other ingredients are lactose monohydrate, corn starch, povidone, talc, and magnesium stearate.
– The film coating contains hypromellose, triacetin, and talc.
What the medicine looks like and the contents of the pack
The film-coated tablets are white and round with a diameter of 6 mm. The tablets are marked NOVO 288 on one side and have a Novo Nordisk logo (Apistjur) on the other.
Pack sizes:
- 1 x 28 film-coated tablets in a calendar pack
- 3 x 28 film-coated tablets in calendar packs
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Novo Nordisk A / S
Novo Allé
DK-2880 Bagsværd
Denmark
This medicinal product is authorized under the European Economic Area under the names:
Activelle (excluding the UK)
United Kingdom: Kliovance
This leaflet was last modified on 25/04/2016
Other sources of information
Further information about this medicine can be found on the Medical Products Agency’s website.
Instructions for use
How to use calendar packaging one
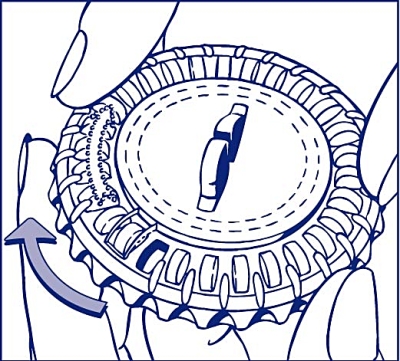
Set the day of the week
Turn the inner disc to set the day of the week opposite the small plastic hub.
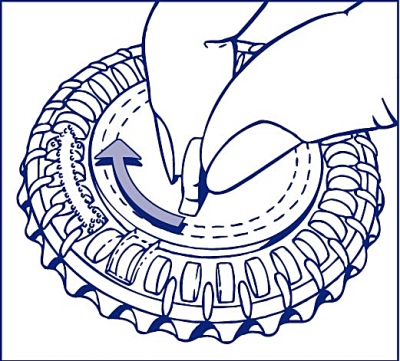
2. How to take out the first tablet
Break off the plastic hub and tip out the first tablet.
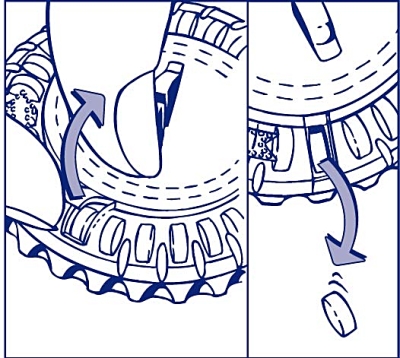
3. Turn the lid every day
Turn the transparent lid clockwise one step in the direction of the arrow the next day. Tip out the next tablet. Remember to only take 1 tablet daily.
The transparent lid can only be rotated after the tablet in the opening has been removed.