What Soolantra is and what it is used for
Soolantra contains the active substance ivermectin which belongs to a group of medicines called avermectins. Cream is used on the skin to treat the rashes and bumps that occur in rosacea.
Soolantra should only be used by adults (18 years of age or older).
What you need to know before you use Soolantra
Do not use Soolantra:
- if you are allergic to ivermectin or any of the other ingredients of this medicine (listed in section 6).
Warnings and precautions
Talk to your doctor or pharmacist before using Soolantra.
At the beginning of treatment, some patients may experience worsening rosacea symptoms, but this is unusual and usually resolves within 1 week of treatment. Talk to your doctor if this happens.
Other medicines and Soolantra
Other medicines could affect Soolantra and you should therefore tell the doctor if you are using, have recently used, or might be using other medicines.
Pregnancy and breastfeeding
Soolantra is not recommended during pregnancy.
If you are breastfeeding, you should not use this medicine, or stop breastfeeding before starting treatment with Soolantra. Talk to your doctor and he or she can help you decide between using Soolantra and breastfeeding, taking into account the benefit of treatment and the benefit of breastfeeding.
Driving ability and use of machinery
Soolantra has no or negligible effect on the ability to drive and use machines.
Soolantra contains:
- Cetyl alcohol and stearyl alcohol can cause local skin irritations (for example contact dermatitis),
- Methyl parahydroxybenzoate (E218) and propyl parahydroxybenzoate (E216) can cause allergic reactions (possibly delayed),
- Propylene glycol can cause skin irritation.
How to use Soolantra
Always use this medicine as directed by your doctor. Ask your doctor or pharmacist if you are unsure.
Important: Soolantra is intended for adults and use on the skin of the face only. Do not use this medicine on other parts of the body, especially on moist body surfaces such as the eyes, mouth, or mucous membranes. Should not be swallowed.
The recommended dose is one application per day on the skin of the face. Apply a pea-sized amount of cream to each of the five areas of the face: forehead, chin, nose, and each cheek. Then spread the cream in a thin layer all over the face.
Be careful to avoid the eyelids, lips, and mucous membranes such as the inside of the nose, mouth, and eyes. If you accidentally get cream in your eyes or near your eyes, eyelids, lips, mouth, or mucous membranes, rinse immediately with plenty of water.
Do not use cosmetics (other face creams or make-up) before applying the daily dose of Soolantra. Such products can be used when the cream has dried.
Wash your hands immediately after applying the cream.
You must use Soolantra daily during treatment. The treatment course can be repeated. The doctor will tell you how long to use Soolantra. The treatment time can vary from person to person and depends on how serious the skin problems are.
You may see an improvement after 4 weeks of treatment. If the condition has not improved after 3 months, discontinue treatment and consult a doctor.
Impaired liver function
If you have liver problems, consult a doctor before using Soolantra.
Use for children and adolescents
Soolantra should not be used by children and adolescents.
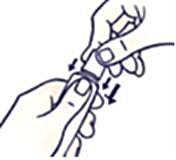
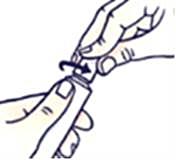
How to open the tube with the childproof cap
To avoid spillage, do not press on the tube when opening or closing it.
Press down on the cap and turn counterclockwise (turn to the left). Then pull off the cork.
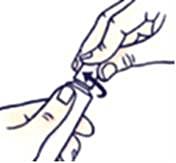
How to close the tube with the childproof cap
Press the cap down and turn clockwise (turn to the right).
If you have used too much Soolantra
If you have ingested too much medicine or if, for example, a child has ingested the medicine by mistake, contact a doctor or hospital for an assessment of the risk and advice.
If you use more than the recommended daily dose, contact your doctor who will tell you what to do.
If you forget to use Soolantra
Do not use a double dose to make up for a missed dose.
If you stop using Soolantra
Several applications of this medicine are needed before the rash and warts begin to diminish. You must continue to use Soolantra for as long as your doctor has prescribed.
If you have any further questions about this medicine, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Soolantra can cause the following side effects:
Common side effects (may affect up to 1 in 10 users)
- Burning sensation in the skin
Uncommon side effects (may affect up to 1 in 100 users)
- Skin irritation
- Itchy skin
- Dry skin
- Worsening of rosacea (consult your doctor)
Frequency not known (cannot be calculated from available data)
- Skin redness
- Skin inflammation
- Swelling of the face
- Elevated liver enzymes (ALT/AST)
How to store Soolantra
Keep this medicine out of the sight and reach of children.
Use before the expiry date which is stated on the carton and tube after EXP. The expiration date is the last day of the specified month.
Once the tube has been opened, the medicine must be used within 6 months.
No special storage instructions.
Unused Soolantra cream should not be thrown down the drain or among the household waste. Ask the pharmacist how to dispose of medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
- The active substance is ivermectin. One gram of cream contains 10 mg of ivermectin.
- Other ingredients are glycerol, isopropyl palmitate, carbomer, dimethicone, disodium edetate, citric acid monohydrate, cetyl alcohol, stearyl alcohol, macrogol cetostearyl ether, sorbitan stearate, methyl parahydroxybenzoate (E218), propyl parahydroxybenzoate (E216), phenoxyethanol, propylene glycol, oleyl alcohol, sodium hydroxide, purified water.
Appearance and package sizes of the medicine
Soolantra is a white to pale yellow cream. It is provided in tubes containing 2, 15, 30, 45, or 60 grams of cream. The larger tubes have a childproof cap, which the 2g tube does not.
Packaging with 1 tube.
Not all pack sizes may be marketed.
This medicine is approved within EEA member states under the following names:
| Austria, Germany, Portugal: | Soolantra 10 mg/g Cream |
| Belgium, Luxembourg: | Soolantra 10 mg/g creamSoolantra 10 mg/g Cream |
| Bulgaria: | Soolantra 10 mg/g Cream |
| Cyprus, Greece: | Soolantra 10 mg/g Κρέμα |
| Czech Republic, Hungary, Slovakia: | Soolantra 10 mg/g cream |
| Denmark: | Soolantra |
| Estonia: | Soolantra 10 mg/g cream |
| Finland: | Soolantra 10 mg/g emulsiovoid |
| France, Netherlands: | Soolantra 10 mg/g cream |
| Iceland, Norway, Poland: | Soolantra 10 mg/g cream |
| Ireland, United Kingdom: | Soolantra 10 mg/g cream |
| Italy: | Efacti 10 mg/g crema |
| Latvia: | Soolantra 10 mg/g cream |
| Lithuania: | Soolantra 10 mg/g cream |
| Malta: | Soolantra 10 mg/g cream |
| Romania: | Soolantra 10 mg/g Cream |
| Spain: | Soolantra 10 mg/g crema |
| Sweden: | Soolantra 10 mg/g cream |
Manufacturer
Laboratoires Galderma
ZI – Montdésir
74 540 Alby sur Chéran
France