5 mg, 10 mg, 15 mg, 20 mg, 30 mg, 40 mg, 60 mg, 80 mg prolonged-release tablet is
oxycodone hydrochloride
What Oxikodon Depot Actavis is and what it is used for
Oxycodone Depot Actavis contains the active substance oxycodone hydrochloride, which belongs to a group of medicines called opioids. These are strong painkillers.
Oxycodone Depot Actavis is used to treat severe pain that can only be alleviated with enough painkillers opioid drugs in adults and children from 12 years of age.
Oxycodone hydrochloride contained in Oxycodone Depot Actavis may also be approved for the treatment of other conditions not mentioned in this product information. Ask your doctor, pharmacist, or other healthcare professionals if you have any further questions, and always follow their instructions.
What you need to know before you use Oxikodon Depot Actavis
Do not use Oxycodone Depot Actavis
- if you are allergic to oxycodone hydrochloride or any of the other ingredients of this medicine (listed in section 6).
- if you have severe respiratory distress ( respiratory depression ) with too low oxygen levels in the blood ( hypoxia ) and/or too high carbon dioxide concentrations in the blood.
- if you have severe chronic obstructive pulmonary disease (COPD), cor pulmonale (changes in the heart due to chronic congestion of the pulmonary circulation), or acute, severe asthma.
- if you have intestinal upset.
- if you have acute severe abdominal pain or delayed emptying of the stomach.
Warnings and cautions
Talk to your doctor or pharmacist before taking Oxikodon Depot Actavis:
- if you are elderly or debilitated.
- if your lung, liver, or kidney function is severely impaired.
- if you have thyroid disease or impaired thyroid function.
- if you have impaired adrenal function, e.g. Addison’s disease.
- if you have an enlarged prostate.
- if you are an alcoholic or undergoing treatment for alcohol dependence.
- if you know you are addicted to opioids.
- if you have inflammation of the pancreas.
- in conditions with increased pressure in the brain, e.g. in the event of a head injury.
- if you have circulatory disorders.
- if you have colic in the bile duct and ureter.
- if you have low blood pressure or low blood volume.
- if you have epilepsy or a tendency to seizures.
- if you are taking MAOIs (to treat depression).
- if you have an inflammatory bowel disease.
- if you have recently undergone abdominal surgery.
Tell your doctor if any of the above apply to you or if you have had any of the above before.
Oxycodone Depot Actavis has primary addiction potential. When used for a long time, tolerance to the effects may mean that an increased dose is required to maintain pain relief.
Chronic use of Oxycodone Depot Actavis may lead to physical dependence and withdrawal symptoms may occur with abrupt discontinuation of treatment. When a patient no longer needs oxycodone treatment, it is advisable to reduce the dose gradually to prevent withdrawal symptoms.
The risk of developing physical or mental dependence is small when the drug is used as prescribed by patients suffering from chronic pain, and this risk must be weighed against the possible benefit. Discuss this with your doctor.
An increased sensitivity to pain, which does not respond to a dose increase of oxycodone, may occur during treatment with Oxycodone Depot Actavis. This is unusual, but if it does, your doctor may reduce your dose or switch to another opioid.
Oxycodone Depot Actavis is not recommended before surgery or up to 24 hours after.
Oxycodone Depot Actavis should be used with special caution by people with a history of alcohol or drug addiction.
Like other opioids, Oxikodon Depot Actavis can affect the normal production of hormones in your body, such as cortisol or sex hormones. This is especially true if you are taking high doses for a long time. Symptoms may include nausea or vomiting, loss of appetite, fatigue, dizziness, altered sexual ability, changes in the menstrual cycle, or impotence. Discuss this with your doctor.
Children and young people
Oxycodone has not been studied in children under 12 years of age. Safety and efficacy have not been established and therefore use in children under 12 years of age are not recommended.
Elderly patients
In elderly patients without renal and/or hepatic impairment, no dose adjustment is usually required.
Other medicines and Oxikodon Depot Actavis
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
If you take these tablets with other medicines, the effect of these tablets or the other medicine may increase or decrease. The risk of side effects can also increase. Tell your doctor or pharmacist if you are taking:
- a type of medicine called a monoamine oxidase inhibitor ( MAOIs, such as moclobemide, phenelzine, isoniazid, tranylcypromine, or selegiline ), or if you have taken this type of medicine in the last two weeks (see section “Warnings and precautions”),
- medicines that help you sleep or stay calm (eg hypnotics or sedatives, including benzodiazepines ),
- medicines used to treat depression (eg paroxetine or fluoxetine ),
- medicines used to treat psychiatric or mental illnesses (such as phenothiazines or neuroleptic medicines),
- other strong painkillers ( opioids ),
- muscle relaxants,
- quinidine (a drug used to treat rapid heartbeat),
- cimetidine (a medicine used to treat stomach ulcers, indigestion, or heartburn),
- medicines used to treat fungal infections (such as ketoconazole , voriconazole, itraconazole or posaconazole),
- drugs used to treat bacterial infections (such as clarithromycin, erythromycin, or telithromycin),
- a specific drug called protease inhibitor used to treat HIV (eg boceprevir, ritonavir, indinavir, nelfinavir, saquinavir),
- rifampicin to treat tuberculosis,
- carbamazepine (a medicine for seizures, seizures, tremors, and certain pain conditions),
- phenytoin (a medicine for seizures, seizures, and tremors),
- St. John’s wort, a traditional herbal medicine (Hypericum Perforatum),
- medicines used to treat allergies ( antihistamines ) or vomiting (antiemetics),
- medicines used to treat Parkinson’s disease,
- coumarin-type anticoagulants (medicines that reduce the blood’s ability to clot).
You should also tell your doctor if you have recently been anesthetized.
Concomitant use of Oxycodone Depot Actavis with sedatives or medicines for sleep disorders such as benzodiazepines or similar medicines increases the risk of drowsiness, difficulty breathing ( respiratory depression ), coma and may be life-threatening. Due to this, concomitant use should only be considered when other treatment options are not possible.
If your doctor prescribes Oxikodon Depot Actavis at the same time as sedatives, the dose and treatment time should be limited by your doctor.
Tell your doctor if you are taking any sedatives and carefully follow your doctor’s dose recommendations. It may be helpful to inform friends or relatives about paying attention to the signs and symptoms described above. Contact a doctor if you experience any of these symptoms.
The risk of side effects is increased if you take antidepressant drugs (for example, citalopram, duloxetine, escitalopram, fluoxetine, fluvoxamine, paroxetine, sertraline, venlafaxine ). These drugs can affect or be affected by oxycodone, and you may experience symptoms such as involuntary, rhythmic muscle contractions, including in the muscles that control eye movements, sudden movements, heavy sweating, tremors, excessive reflexive movements, increased muscle tension, body temperature above 38 ° C. Contact your doctor if you experience these symptoms.
Oxycodone Depot Actavis with food, drink, and alcohol
You should not drink alcohol while taking Oxikodon Depot Actavis. Drinking alcohol while taking Oxikodon Depot Actavis may make you feel sleepy or increase the risk of serious side effects such as weak breathing with the risk of respiratory arrest and unconsciousness.
Grapefruit juice can inhibit the breakdown of oxycodone which leads to an increased effect. Therefore, you should avoid drinking grapefruit juice while taking Oxikodon Depot Actavis.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
There is insufficient experience with oxycodone treatment in pregnant women. Oxycodone passes through the placenta into the bloodstream of the fetus.
The use of oxycodone during pregnancy may cause withdrawal symptoms in the newborn. Newborns of mothers who have received oxycodone during the last 3 to 4 weeks before delivery is at risk of severe respiratory distress ( respiratory depression ). The use of oxycodone during labor can cause severe breathing problems in the newborn.
Oxycodone Depot Actavis should only be used during pregnancy if the benefit outweighs the risk to the baby.
Breast-feeding
You should not take Oxycodone Depot Actavis during the breastfeeding period as oxycodone may pass into breast milk and may cause breathing difficulties in the newborn baby.
Driving and using machines
Oxycodone can affect the ability to drive or use machines.
During stable treatment, it is not necessary to ban driving in general. The treating physician must evaluate the individual situation. Discuss with your doctor if and under what circumstances you can drive.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires sharpened attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. The description of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
Oxycodone Depot Actavis contains lactose
This medicine contains lactose. If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
How to take Oxycodone Depot Actavis
Use for children and adolescents
Always take this medicine exactly as your doctor or pharmacist has told you. Ask your doctor or pharmacist if you are unsure.
Adults and adolescents (12 years or older)
The usual starting dose is 5 or 10 mg oxycodone hydrochloride at 12-hour intervals. Your doctor will prescribe the dose needed to treat the pain.
Further determination of the daily dose one, the division of the doses, and all dose adjustments during treatment are carried out by the treating physician and depend on the previous dose one.
Patients who have previously taken opioids can start treatment with higher doses, taking into account their previous experience with opioid treatment.
Some patients receiving Oxikodon Depot Actavis on an established schedule need fast-acting analgesics such as emergency medicine to control breakthrough pain. Oxycodone Depot Actavis is not indicated for the treatment of breakthrough pain.
In the treatment of non-cancer-related pain, a dose of 20 mg oxycodone hydrochloride twice daily is usually sufficient, but a higher dose may be needed. Patients with cancer pain usually need higher doses, from 80 mg to 120 mg oxycodone hydrochloride, which can be increased to 400 mg oxycodone hydrochloride in individual cases.
The treatment needs to be checked regularly about pain relief and other effects to achieve the best possible pain treatment as well as the possibility of treating all side effects that occur in good time and deciding whether to continue the treatment.
Impaired kidney or liver function or low body weight
If you have impaired kidney and/or liver function or if you have low body weight, your doctor may prescribe a lower starting dose.
How to take the medicine and the duration of treatment
Swallow the prolonged-release tablet whole with a sufficient amount of liquid (½ glass of water), with or without food, in the morning and evening according to a set schedule (eg 8.00 am and 8.00 pm).
The prolonged-release tablet must not be broken, crushed, or chewed as this will lead to rapid release of oxycodone due to damage to the tablet’s sustained-release properties. If you swallow a prolonged-release or chewed prolonged-release tablet, this will lead to rapid release and absorption of a lethal dose of oxycodone (see section “If you have taken too much Oxycodone Depot Actavis”).
Oxycodone Depot Actavis prolonged-release tablets are for oral use only. In the case of drug injection ( injection into a vein), the ingredients of the tablet may lead to degeneration ( necrosis ) of the nearby tissue, alteration of lung tissue (lung granuloma), or other serious events that can be fatal.
Instructions for use of child-resistant blister packs:
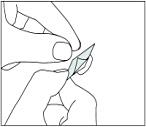
1. Do not attempt to push the tablet directly out of the tablet compartment. You can not push the tablet out through the foil. Instead, the foil must be removed.
2. First separate a dose from the rest of the strip at the perforations.
Then gently pull off the back to open the tablet compartment.
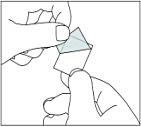
4. You can then take the tablet out of the tablet compartment.
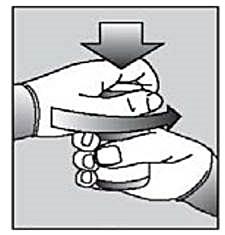
Instructions for use of child protection cans:
Press the lid down and turn it to open.
Your doctor will adjust the dose depending on the intensity of the pain and how you respond to the treatment. Take the number of prolonged-release tablets prescribed by your doctor twice a day.
If you take more Oxikodon Depot Actavis then you should
If you have ingested too much medicine or if e.g. If a child has ingested the medicine by mistake, contact a doctor or hospital for risk assessment and advice.
The following symptoms may occur: constricted pupils, decreased breathing, muscle weakness, drowsiness, and drop in blood pressure. In severe cases, circulatory collapse, mental and motor inactivity, unconsciousness, slow heartbeat and accumulation of water in the lungs, low blood pressure, or death may occur. Abuse of high doses of strong opioids such as oxycodone can be fatal. Under no circumstances should you be exposed to situations that require a high ability to concentrate, such as driving.
If you forget to take Oxikodon Depot Actavis
If you take a smaller dose of Oxikodon Depot Actavis than you were instructed to take or if you forget to take a tablet, the pain relief will be insufficient or stop completely.
You can compensate for a forgotten tablet if the next planned regular intake is not to take place until at least 8 hours. Then you can continue to take the tablets according to the instructions.
You should also take prolonged-release tablets if the time to the next regular intake is shorter, but then you should postpone the next intake by 8 hours. In principle, you should not take Oxikodon Depot Actavis more often than every 8 hours.
Do not take a double dose to make up for a forgotten tablet.
If you stop taking Oxikodon Depot Actavis
Do not stop treatment without talking to your doctor.
When a patient no longer needs treatment with Oxikodon Depot Actavis, it is advisable to reduce the dose gradually to prevent withdrawal symptoms.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If you experience any of the following side effects, stop using Oxikodon Depot Actavis immediately and contact your doctor or go to the nearest emergency room:
- Respiratory depression (shallow breathing) is the most serious risk caused by opioids and is most likely to occur in elderly or debilitated patients. This can also lead to a sharp drop in blood pressure.
- severe allergic reactions (anaphylactic shock ) which may cause hives, swelling of the face, lips, mouth, tongue, or throat, or difficulty breathing.
- reduction in pupil size
- sudden difficulty breathing ( bronchospasm )
- abdominal cramps ( spasm in the smooth muscles around the intestines)
- subdued cough reflex.
Other possible side effects are
Very common (may affect more than 1 user in 10):
- decreased level of consciousness (fatigue to drowsiness). This usually occurs at the beginning of your treatment or if you increase the dose one and usually disappear after a few days.
- dizziness.
- headache.
- constipation.
- nausea.
- vomiting.
- itching.
Common (may affect up to 1 in 10 people):
- a feeling of weakness ( asthenia )
- various psychological side effects are such as:
- mood swings (such as anxiety, depression).
- altered activity level (nervousness and sleep disorders).
- altered behavior (thought disorders, confusion, memory loss, isolated cases of speech disorders).
- tremors or involuntary tremors.
- shortness of breath.
- difficulty breathing or wheezing.
- dry mouth, in rare cases together with thirst, stomach problems such as stomach pain, diarrhea, indigestion, decreased appetite.
- skin problems such as rash, in rare cases, increased sensitivity to sunlight ( photosensitivity ), in some cases itching or scaly rash, sweating.
- problems urinating (increased need to urinate).
Uncommon (may affect up to 1 in 100 people):
- allergic reactions.
- dehydration.
- the feeling of anxiety or upset.
- emotional fluctuations such as unstable emotional state, personality change, an abnormal feeling of happiness and seeing, hearing, or feeling things that are not there (hallucinations), altered taste buds, visual disturbances, unusually sharp hearing, feeling dizzy or dizzy, decreased sex drive; dependent (see section 2).
- abnormal secretion of antidiuretic hormone.
- memory gaps, seizures, difficulty stretching muscles, increased or decreased muscle tone, ticks, decreased sensitivity to touch, coordination difficulties, speech difficulties, fainting, and crawling.
- feeling sick, increased heart rate, awareness of the heartbeat.
- dilated blood vessels.
- cough, sore throat, runny nose, voice changes, difficulty breathing.
- cold sores, inflammation of the gums, inflammation of the mouth, difficulty swallowing, gas, constipation in the intestine.
- elevated liver enzymes.
- dry skin.
- difficulty urinating.
- impaired sexual function, impotence.
- injuries as a result of accidents.
- pain (eg chest pain), too much fluid in the tissues ( edema ), chills, thirst, migraine, physical dependence with withdrawal symptoms.
- changes in tear flow, contraction of the pupils, visual disturbances.
Rare (may affect up to 1 in 1,000 people):
- a disease of the lymph nodes.
- lowering of blood pressure, dizziness when standing up from sitting or lying down.
- muscle spasms (involuntary muscle contractions).
- bleeding gums, increased appetite, dark-colored stools, stains on the teeth.
- herpes simplex (a disease that affects the skin and mucous membranes).
- itchy skin rash (hives).
- blood in the urine.
- changes in body weight (increased or decreased), cellulite ( inflammation of the subcutaneous tissue).
Very rare (may affect up to 1 in 10,000 people):
- severe allergic reactions (anaphylactic reactions).
- aggression.
- increased pain sensitivity which is not improved by increased dosage.
- caries.
- pain on the right side of the stomach, itching, and jaundice caused by inflammation of the gallbladder.
- menstrual failure.
- Long-term use of Oxycodone Depot Actavis during pregnancy may cause life-threatening withdrawal symptoms in newborns. Symptoms to look out for are e.g. irritability, hyperactivity and abnormal sleep patterns, screams, tremors, vomiting, diarrhea, and lack of weight gain.
Prevention:
If you experience any of the side effects mentioned above, your doctor will usually take the necessary measures. Side effects in the form of constipation can be prevented through a fiber-enriched diet and increased fluid intake. If you suffer from nausea or vomiting, your doctor will prescribe a suitable medicine for this.
How to store Oxikodon Depot Actavis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the label or carton after EXP. The expiration date is the last day of the specified month.
Blister packs:
Do not store above 25 ° C.
HDPE can :
5 mg, 10 mg, 15 mg: Do not store above 30 ° C.
20 mg, 30 mg, 40 mg, 60 mg, 80 mg: No special storage instructions.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Contents of the pack and other information
Content declaration
- The active substance is oxycodone hydrochloride. Each tablet contains 5, 10, 15, 20, 30, 40, 60 or 80 mg of oxycodone hydrochloride.
- Other ingredients are Tablet core: Lactose monohydrate, hypromellose, povidone, stearic acid, magnesium stearate, colloidal anhydrous silica. Tablet coating:5 mg tablets: polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, blue indigo carmine (E132), yellow iron oxide (E172).10 mg tablets: titanium dioxide (E171), hypromellose, macrogol, polysorbate 80.15 mg tablets: polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, yellow iron oxide (E172), black iron oxide (E172).20 mg tablets: polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, red iron oxide (E172).30 mg tablets: polyvinyl alcohol, macrogol, talc, red iron oxide (E172), black iron oxide (E172), blue indigo carmine (E132).40 mg tablets: polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, yellow iron oxide (E172).60 mg tablets: polyvinyl alcohol, macrogol, talc, red iron oxide (E172), black iron oxide (E172), blue indigo carmine (E132).80 mg tablets: polyvinyl alcohol, titanium dioxide (E171), macrogol, talc, blue indigo carmine (E132), yellow iron oxide (E172).
What the medicine looks like and the contents of the pack
Oxycodone Depot Actavis 5 mg prolonged-release tablets are blue, round, biconvex tablets, 7 mm in diameter marked with “OX 5” on one side.
Oxycodone Depot Actavis 10 mg prolonged-release tablets are white, round, biconvex tablets, 9 mm in diameter marked with “OX 10” on one side.
Oxycodone Depot Actavis 15 mg prolonged-release tablets are gray, round, biconvex tablets, 9 mm in diameter marked with “OX 15” on one side.
Oxycodone Depot Actavis 20 mg prolonged-release tablets are pink, round, biconvex tablets, 7 mm in diameter marked with “OX 20” on one side.
Oxycodone Depot Actavis 30 mg prolonged-release tablets are brown, round, biconvex tablets, 9 mm in diameter marked with “OX 30” on one side.
Oxycodone Depot Actavis 40 mg prolonged-release tablets are yellow, round, biconvex tablets, 7 mm in diameter marked with “OX 40” on one side.
Oxycodone Depot Actavis 60 mg prolonged-release tablets are red, round, biconvex tablets, 9 mm in diameter marked with “OX 60” on one side.
Oxycodone Depot Actavis 80 mg prolonged-release tablets are green, round, biconvex tablets, 9 mm in diameter marked with “OX 80” on one side.
Oxycodone Depot Actavis is available in blister packs for:
5 mg: 1, 20, 28, 30, 50, 56, 60 and 100 prolonged-release tablets .
10 mg, 20 mg, 40 mg, 80 mg: 1, 20, 28, 30, 50, 56, 60, 98 and 100 prolonged-release tablets .
15 mg: 1, 20, 28, 30, 50, 56, 98 and 100 prolonged-release tablets .
30 mg, 60 mg: 1, 20, 28, 30, 50, 56, 98 and 100 prolonged-release tablets .
Oxycodone Depot Actavis is available in child-resistant blister packs for:
5 mg: 1, 20, 28, 30, 50, 56, 60 and 100 prolonged-release tablets .
10 mg, 20 mg, 40 mg, 80 mg: 1, 20, 28, 30, 50, 56, 60, 98 and 100 prolonged-release tablets .
15 mg: 1, 20, 28, 30, 50, 56, 98 and 100 prolonged-release tablets .
30 mg, 60 mg: 1, 20, 28, 30, 50, 56, 98 and 100 prolonged-release tablets .
Oxycodone Depot Actavis is also available in white, round, child-resistant HDPE jars with PP lids containing 98 or 100 prolonged-release tablets.
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Actavis Group PTC EHF
Reykjavíkurvegur 76 78
220 Hafnarfjörður
Iceland
Manufacturer
Balkanpharma Dupnitsa AD
3 Samokovsko Shosse Str.
Dupnitsa 2600
Bulgaria