 |
NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials |
| << SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS |
| THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible >> |
Chapter
9
NEWER
SYNTHETIC STRATERGIES FOR
NANOMATERIALS
P.
Sangeetha
1.
INTRODUCTION
The
emerging science in the
world today mainly focuses on
nanomaterials and its
applications.
Nano is interrelated to chemistry,
physics and biology and it has
attracted
the
attention of all eminent
scientists to its side.
Nanochemistry can be described as
a
special
discipline of inorganic or solid
state chemistry. It focuses on the
synthesis of
nanoparticulate
systems. The nanochemist can be considered to
work towards this
goal
from
the atom "up", whereas the
nanophysicist tends to operate from
the bulk "down". It
is
schematically represented in Fig.
9.1
Fig.
9.1 Schematic diagram
1.1
What is Nano?
The
term nano is a measurement of size. A
nanometre (nm) is a millionth of a
millimetre.
By
way of illustration, a nanometer is
about 1/50,000th the width
of a human hair, and a
sheet
of normal office paper is
about 100,000 nm thick. A nanomaterial or
a nanoparticle
is
usually considered to be a structure
between 0.1 and 100 nm (1/1,000,000
mm). The
origin
of the prefix "nano" is from
the Greek word "nanos"'
meaning dwarf.
Nanotechnology
deals with a scale that is
over 10,000 times smaller
than a millimetre. It

9.2
Newer
Synthetic Strategies for
Nanomaterials
involves
investigating, producing and applying
structures that are smaller
than 100
nanometres
(nm). At the nanoscale, the
physical, chemical, and biological
properties of
materials
differ in fundamental and often
valuable ways from the
properties of individual
atoms
and molecules or bulk matter.
Research and development in
nanotechnologies is
directed
toward understanding and creating
improved materials, devices, and systems
that
exploit
these new properties. These properties
have been found to be very
useful for an
increasing
number of commercial applications,
for example: protective
coatings, light-
weight
materials, self-cleaning clothing, to
name but a few. The main
classes of
nanoscale
structures and tunable properties are
summarized in Table 9.1 and
9.2.
Table
9.1 Main classes of
nanoscale:
Dimension
3
dimensions < 100nm
Particles,
quantum dots, hollow
spheres, etc.
2
dimensions < 100nm
Tubes,
fibres, wires, platelets,
etc.
1
dimension < 100nm
Films,
coatings, multilayer,
etc.
Phase
composition
Single-phase
solids
Crystalline,
amorphous particles and layers,
etc.
Multi-phase
solids
Matrix
composites, coated particles,
etc.
Multi-phase
systems
Colloids,
aerogels, Ferro fluids,
etc.
Manufacturing
process
Gas
phase reaction
Flame
synthesis, condensation, CVD,
etc.
Liquid
phase reaction Sol-gel,
precipitation, hydrothermal processing
etc.
Mechanical
properties
Ball
milling, plastic deformation
etc.
Synthetic
Strategies in Chemistry
9.3
Table
9.2. Tunable properties by nanoscale
surface design and their application
potentials
Mechanical
properties (e.g., hardness,
Wear
protection of machinery and
equipment,
scratch
resistance, tribology).
mechanical
protection of soft
materials
(polymers,
wood, textiles, etc.)
Wetting
properties (e.g. anti-adhesive,
Anti-graffiting,
anti-fouling, lotus-effect,
self
hydrophobic,
hydrophilic).
cleaning
surface for textiles and ceramics,
etc.
Corrosion
protection for machinery
and
Thermal
and chemical properties
(e.g.
heat
resistance and insulation, corrosion
equipment, heat resistance for turbines
and
engines,
thermal insulation equipment
and
resistance).
building
materials etc.
Biological
properties (biocompatibility,
Biocompatible
implants, medical tools
and
anti-infective).
wound
dressings etc.
Electronical
and magnetic properties
Ultra-thin
dielectrics for field-effect
transistors,
(
e.g. magnetoresistance, dielectric)
magneto-resistive
sensors and data memory
etc.
Optical
properties (e.g. anti-reflection,
Photo
and electro-chromic windows,
anti-
photo
and electro-chromatic)
reflective
screens and solar cells
etc.
1.2
Nanotechnology:
Nanotechnology
is one of the key technologies of
the 21st century. Nanotechnology
refers
broadly
to a field of applied science and
technology whose unifying
theme is the control
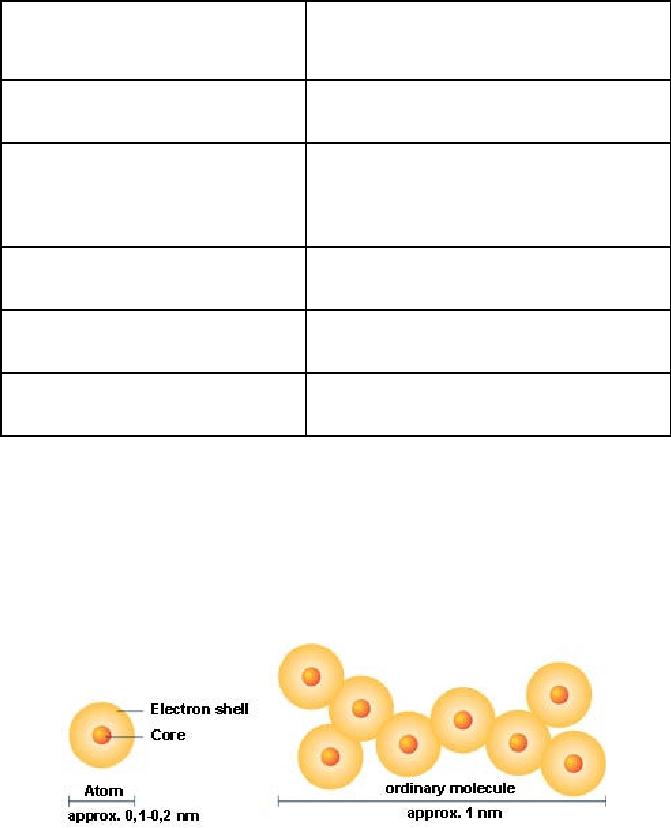
of
matter on the atomic and
molecular scale as shown in
Fig. 9.2, generally
100
nanometers
or smaller, and the fabrication of
devices with critical dimensions
that lie
within
this size range.
Fig.
9.2. Schematic representation
for atom and molecule
9.4
Newer
Synthetic Strategies for
Nanomaterials
1.3
Nanomaterials:
It
is the study of how
materials behave when their
dimensions are reduced to
the
nanoscale.
It can also refer to the materials
themselves that are used in
nanotechnology.
Unique
changes occur in the
electronic structure and chemical
properties of solids
when
their
dimensions are reduced to the nanoscale,
including development of discrete
electronic
structure, quantum confinement,
structural strain and distortion, and
altered
interfacial/surface
reactivity. These changes translate
into new physical and
chemical
behavior
which is not observed in the
`bulk' form of the
material.
Given
that processes at the
gas/solid and liquid/solid interfaces
are largely
controlled
by
local phenomena (e.g., the
availability, reactivity and density of
surface sites), changes
in
the local electronic and
physical structures of a solid
brought about by
`nanostructuring'
which should have a
tremendous impact on heterogeneous
chemistry
(catalysis).
Examples: Au for selective
oxidation, arene and alkene
hydrogenation by Ir,
Rh,
photocatalytic activity of TiO2 and
unique reactivity of oxide
nanoparticles.
2.
NEWER SYNTHETIC STRATEGIES TO BUILD
PROTEIN
BASED
NANOMATERIALS:
Recent
progress in nanotechnology has
yielded new device
components with
unprecedented
capabilities. However, the
small size of these building
blocks makes it
difficult
to position them into
functional assemblies using
existing patterning
techniques.
(a)
(b)
Fig.
9.3 (a) Arrys of
chromophores for light
harvesting and (b) Spherical
carriers for
PET
imaging applications
Synthetic
Strategies in Chemistry
9.5
3.
SYNTHESIS AND CHARACTERIZATION OF
INORGANIC
NANOMATERIALS
As
one solution to this problem,
the protein shells of two
viruses are converted
into
scaffolds
that can position nanoscale objects
with excellent spatial
resolution. In one case,
this
strategy has been used to
synthesize arrays of fluorescent
molecules, providing
efficient
mimics of the light
harvesting system present in
photosynthetic organisms. In a
second
research area, well-defined
core/shell materials have
been prepared for
applications
in diagnostic imaging. The
cornerstone of these efforts
has been a series of
new
synthetic reactions that can
modify biomolecules (Fig.
9.3) with high
site-selectivity
and
yield. The synthetic route
will focus on the development of
the methods and the
applications
of the new materials that
have been built through
their use.
The
study of nanoscale inorganic materials is
an exciting and rapidly growing
area of
research
which offers multiple
opportunities for innovation and
creativity. Our
research
focuses
primarily on the development of
new synthetic approaches to
nanoparticles with
tunable
properties. One aspect of
our work is synthesis of new
precursors as well as
the
application
of established molecular precursors to
synthesize nanometer size metal
alloy,
metal
phosphide, metal oxide, and
metal chalcogenide materials.
Some new wet-
chemistry
procedures to produce size- and
morphology-controlled nanoparticles
are:
Single-source
precursors to heterometallic oxide
materials
Single-source
precursors to phosphide
nanoparticles
Single-source
precursors to metal chalcogenide
materials
Synthesis
of nanoalloys from organometallic
precursors
Shape
control syntheses of iron and manganese
oxides
3.1
Single-Source Precursors to
Heterometallic Oxide
Materials
The
development of efficient photocatalytic
systems has been of vital
interest since these
can
contribute to the reduction of
pollution and solve some
energy-related problems.
However
the synthesis of environmentally-friendly
binary oxides of desired
morphology
still
remains a challenge. In the
synthesis described so far,
the problems
encountered
were
phase inhomogenities and the
inclusion of carbon originating
from the ligands.
Developing
new heterometallic (single-source)
precursors to synthesize AMO3 (A
= Li,
Na,
K, Rb; M = Nb, Ta) and
MBiO4 (M=V,
Nb, Ta) nanoparticles, which
are known as

9.6
Newer
Synthetic Strategies for
Nanomaterials
stable
and efficient photocatalysts. The
precursors to be synthesized are
composed of
salicylate
and alkoxide complexes that
contain the metal elements
in the desired ratios.
A
number
of heterometallic complexes were
synthesized (Fig. 9.4) and
their utility as
single-source
precursors was tested. Pyrolysis and
wet-chemistry routes
were
successfully
used to produce mixed oxide
of micron and nano-sized
particles.
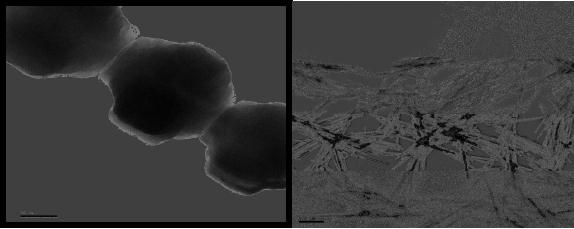
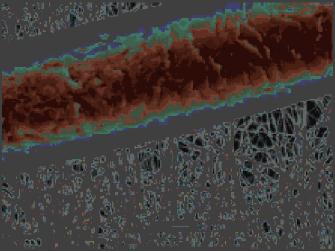
Fig.
9.4 Bi2Ta2(sal)4(Hsal)4(OEt)2 (left)
and SEM image hydrolysis product
(right)
(sal
= O2CC6H4O,
Hsal = O2CC6H4OH)
[12]
3.2
Single-Source Precursors to Phosphide
Nanoparticles
Magnetic
materials can be made in a variety of
forms including nanoparticles,
thin
molecular
films, and bulk
materials.
Fig.
9.5. Iron phosphide nanorods
[13]
Synthetic
Strategies in Chemistry
9.7
Improving
the current technologies
that utilize magnetic
compounds depends upon
the
ability
to produce pure materials; of
concern in the synthesis of
these materials is
the
ability
to produce materials with a
controlled stoichiometry. While
most studies of
magnetic
nanoparticles have been on
magnetic metals, alloys, and
oxides, the focus of
this
research is on binary magnetic
materials composed of transition
metals and p-block
elements.
3.3
Single-Source Precursors to Metal
Chalcogenide Materials
The
initial research will involve
the synthesis of iron
phosphide nanomaterials
from
known
heterometallic precursors; there
are a few binary phases of
iron phosphide that
possess
magnetic properties (FeP
possesses antiferromagnetic behavior,
while Fe3P
and
Fe2P
exhibit ferromagnetic properties).
Fig. 9.5 shows iron
phosphide nanorods
synthesized,
utilizing a heterometallic
iron-phosphorus cluster.
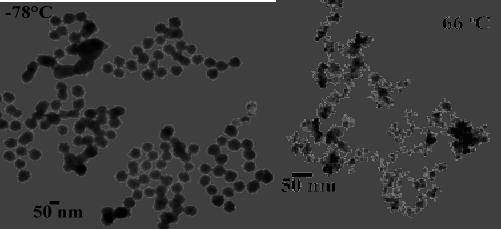
50
nm
Fig.
9.6. Nanoparticles of PbS and
Bi2S3 [14]
The
design
of chalcogenide materials in discrete
forms is a major problem for
modern
solid-state
chemists and materials scientists.
Many of these chalcogenide
materials have
potential
applications in electronic devices. It is
mainly focused on the design,
syntheses,
and
decomposition of new molecular
precursors to such chalcogenide
materials in forms,
such
as rods, wires, and more
complex shapes that have
been unattainable
with
conventional
solid-state methods. Developing
the chemistry of metal
complexes with
chalcogen
containing ligands as molecular
precursors to a number of metal
chalcogenide
materials,
including Bi2S3,
Bi2Se3, PbS, PbSe,
CdS, CdSe (Fig. 9.6). A
major emphasis
of
the synthesis is to control
the organization of the
materials at the nanoscale and to
find
9.8
Newer
Synthetic Strategies for
Nanomaterials
a
relationship between the
nature of the precursors and
the quality of the
nanomaterials
obtained.
To accomplish this, an interdisciplinary
approach, with experience in
synthetic
and
materials chemistry, as well as
crystallography, will provide a plethora
of
opportunities
to investigate these interesting
problems in nanoscience. Various
methods
are
being developed to synthesize, re
shape and study the assembly
characteristics of
chalcogenide
metal nanoparticles.
3.4
Synthesis of Nanoalloys from
Organometallic Precursors
Another
research in the area of nanoscale
materials concerns the
synthesis of alloy and
intermetallic
nanoparticles from the
corresponding organometallic precursors.
The
preparation
of a series of Bi-Ru, Bi-Pd, and
Bi-Pt intermetallic nanocrystals
using wet-
chemistry
procedures has been achieved
(Fig. 9.7). These methods
are based on the
relatively
low-temperature reaction between
two organometallic precursors in
the
presence
or absence of stabilizing agents.
The most advantageous points of
these
procedures
consist in overcoming the
need of chemical reducing
agents and high
temperature
decomposition. In addition, regular
spherical agglomerates are formed
and
their
size could be tuned by varying
temperature and surfactants.
The
efficiency of this
procedure
is shown by the ability of
the particles to be dried and
redispersed, while
maintaining
their morphology. These nano-alloys
have recently attracted
attention
because
of their activity and selectivity
toward numerous catalytic
reactions such as
dehalogenation,
oxidation of alcohols and aldehydes, as
well as for applications in
fuel
cells.
Fig.
9.7 Bi-Pd nanoparticles
prepared at two different
temperatures [13]

Synthetic
Strategies in Chemistry
9.9
3.5
Shape Control Synthesis of Iron
and Manganese oxides
Bulk
iron oxides and manganese
oxides have many technical
applications due to their
magnetic
and catalytic properties. These
properties can be enhanced by tuning
particle
sizes
within the nanometer scale.
Magnetic nanoparticles (NPs),
for example, have
potential
applications in information storage,
medical imaging, drug
delivery, and water
remediation.
Many applications, such as heterogeneous
catalysis, are enhanced by
the
high
surface area that
nanoparticles possess. However,
enhancement of physical
properties
is not only limited to size
effects. There are a number
of publications on
nanoparticles
with shape-dependent magnetic,
electronic, and optical properties.
Most of
these
studies are on NPs such as
spheres, rods, wires, belts,
and disks, which can be
classified
as `traditional' shapes. The
synthesis, the physical
properties, and the
growth
mechanisms
of `traditional' shapes, including
iron and manganese oxides,
have been
widely
investigated and are well-understood.
However, the formation
mechanism and
physical
properties of non-traditional
multi-branched NPs such as tetrapods,
hexapods,
tripods,
stars, and dumbbells, are
not completely understood and
have led to
investigations
on shape controlled synthesis and to
the development of
non-traditional
growth
mechanism.
A
B
C
D
Fig.
9.8. MnO (A and B) and FeO (C andD) NPs
[13]
The
synthesis of new cross-shaped,
hexapod, and concave-faces cubic
nanoparticles
(NPs)
of MnO, FeO, and Fe1-xMnxO
have been reported. The
main goals of these studies
are
:
1.
To develop simple and inexpensive
synthesis to grow nanoparticles
with new shapes.
9.10
Newer
Synthetic Strategies for
Nanomaterials
2.
To study the growth
mechanisms by observation of the
nanoparticle morphologies as
a
function of the reaction parameters:
surfactant ratio, water
concentration, time,
temperature,
and precursor.
Some
of the morphologies obtained
are shown in Fig.
9.8
A
motivation in nanoscience is to try to
understand how materials behave
when
sample
sizes are close to atomic
dimensions. Fig. 9.8 for
example shows a picture
of
nanofibrils
that are 10 to 100 times
smaller in diameter than
conventional textile fibers.
In
comparison
to a human hair which is ca.
80,000 nm in diameter, the nanofibers
are 1,000
times
smaller in diameter. When
the characteristic length
scale of the microstructure is
in
the
1- 100 nm range, it becomes comparable
with the critical length
scales of physical
phenomena,
resulting in the so-called
"size and shape effects."
This leads to unique
properties
and the opportunity to use
such nanostructured materials in
novel applications
and
devices. Phenomena occurring on this
length scale are of interest
to physicists,
chemists,
biologists, electrical and mechanical
engineers, and computer
scientists,
making
research in nanotechnology a frontier
activity in materials science.
Fig.
9.9 A picture of nanofibrils
shown with a human hair
for reference [17]
On
tracking the nano evolution,
it has been stated that no
matter what the
market
outcomes
in the near or long term, nanoscience
will never be an industry unto
itself but a
science
of many avenues of application, and
possibility that could
redefine the
direction
of
several industries. This
insight allows one to recognize
that nanotechnology is not
"a
Synthetic
Strategies in Chemistry
9.11
technology"
but "a set of technologies,"
yielding a set of technical
breakthroughs that
will
sweep into many different
markets. Within such a
framework, the world
of
nanotechnology
may be divided into three
broad categories: nanostructured
materials,
nanotools,
and nanodevices.
4.
Nanotools
These
include fabrication techniques;
analysis and metrology instruments; and
software
for
nanotechnology research and development.
They are used in
lithography, chemical
vapor
deposition (CVD), 3-D
printing, and nanofluidics. Nanofluidics,
the study of
nanoscale
fluid behavior, for example,
the study of dynamics of
droplets adsorbed
onto
surfaces
under shearing, is mostly
used in areas such as
medical diagnostics and
biosensors.
5.
Nanostructured Materials
Nanocrystalline
Materials
�
Fullerenes/
Carbon Nanotubes
�
Dendrimers
(Organic Nanoparticles)
�
Polyhedral
Silsesquioxanes (Inorganic-Organic Hybrid
Nanoparticles)
�
Nano-Intermediates
�
Nanocomposites
�
Nanostructured
(NsM) materials are
materials with a microstructure
the characteristic
length
scale of which is on the
order of a few (typically
1-100) nanometers.
The
microstructure
refers to the chemical
composition, the arrangement of
the atoms, and the
size
of a solid in one, two, or
three dimensions. Effects
controlling the properties of
nanostructured
materials include size effects
(where critical length
scales of physical
phenomena
become comparable with the
characteristic size of the building
blocks of the
microstructure),
changes of the dimensionality of
the system, changes of the
atomic
structure,
and alloying of components (e.g.,
elements) that are not
miscible in the solid
and/or
the molten state.
The
synthesis, characterization and processing of
nanostructured materials are
part of
an
emerging and rapidly growing
field. Research and developement in
this field
emphasizes
scientific discoveries in the generation
of materials with
controlled
microstructural
characteristics, research on their
processing into bulk materials
with
9.12
Newer
Synthetic Strategies for
Nanomaterials
engineered
properties and technological functions, and
introduction of new
device
concepts
and manufacturing methods.
Nanostructured
materials may be grouped
under nanoparticles (the
building blocks),
nano-intermediates,
and nanocomposites. They may be in or
far away from
thermodynamic
equilibrium. For example,
nanostructured materials consisting
of
nanometer-sized
crystallites of Au or NaCl with
different crystallographic
orientations
and/or
chemical compositions vary
greatly from their
thermodynamic equilibrium.
Nanostructured
materials
synthesized
by
supramolecular
chemistry
yielding
nanoassemblies
are examples of those in thermodynamic
equilibrium. In the subsequent
paragraphs,
the various classes of
nanoparticles that serve as the
building blocks of
nanomaterials
and devices will be discussed. They
include nanocrystalline materials
such
as
ceramic, metal and metal
oxide nanoparticles; fullerenes,
nanotubes and related
structures;
nanofibers and wires, and precise
organic as well as hybrid
organic-inorganic
nanoarchitechtures
such as dendrimers and polyhedral
silsesquioxanes, respectively.
5.1
Nanocrystalline Materials
Ceramics,
metals, and metal oxide
nanoparticles fall in this
category. In the last
two
decades
a class of materials with a
nanometer-sized microstructure have
been
synthesized
and studied. These materials are
assembled from nanometer-sized
building
blocks,
mostly crystallites. The
building blocks may differ
in their atomic
structure,
crystallographic
orientation, or chemical composition. In
cases where the
building
blocks
are crystallites, incoherent or
coherent interfaces may be
formed between them,
depending
on the atomic structure, the
crystallographic orientation, and the
chemical
composition
of adjacent crystallites. In other
words, materials assembled of
nanometer-
sized
building blocks are
microstructurally heterogeneous, consisting of
the building
blocks
(e.g. crystallites) and the
regions between adjacent
building blocks (e.g.
grain
boundaries).
It is this inherently heterogeneous
structure on a nanometer scale
that is
crucial
for many of their properties and
distinguishes them from
glasses, gels that
are
microstructurally
homogeneous.
Grain
boundaries make up a major portion of
the material at nanoscales, and
strongly
affect
properties and processing. The properties of
NsM deviate from those of
single
crystals
(or coarsegrained polycrystals) and
glasses with the same
average chemical
Synthetic
Strategies in Chemistry
9.13
composition.
This deviation results from
the reduced size and dimensionality of
the
nanometer-sized
crystallites as well as from
the numerous interfaces
between adjacent
crystallites.
An attempt is made to summarize
the basic physical concepts and
the
microstructural
features of equilibrium and
non-equilibrium NsM. Nanocrystallites
of
bulk
inorganic solids have been
shown to exhibit size dependent
properties, such as
lower
melting points, higher
energy gaps, and nonthermodynamic
structures. In
comparison
to macro-scale powders, increased
ductility has been observed
in
nanopowders
of metal alloys. In addition,
quantum effects from
boundary values
become
significant leading to such
phenomena as quantum dots
lasers.
One
of the primary applications of
metals in chemistry is their
use as heterogeneous
catalysts
in a variety of reactions. In general,
heterogeneous catalyst activity is
surface
dependent.
Due to their vastly
increased surface area over
macro-scale materials,
nanometals
and oxides are ultra-high
activity catalysts. They are
also used as desirable
starting
materials for a variety of
reactions. Nanometals and oxides
are also widely used
in
the formation of nanocomposites.
Aside from their synthetic
utility, they have
many
useful
and unique magnetic, electric, and
optical properties.
5.2
a. Fullerenes:
The
discovery of fullerenes in 1985 by Curl,
Kroto, and Smalley culminated in
their
Nobel
Prize in 1996. Fullerenes, or
Buckminsterfullerenes, are named after
Buckminster
Fuller
the architect and designer of the
geodesic dome and are sometimes called
bucky
balls.
The names derive from
the basic shape that
defines fullerenes; an elongated
sphere
of
carbon atoms formed by interconnecting
six-member rings and twelve
isolated five-
member
rings forming hexagonal and
pentagonal faces. The first
isolated and
characterized
fullerene, C60, contains 20 hexagonal
faces and 12 pentagonal faces
just
like
a soccer ball and possesses
perfect icosahedral
symmetry.
Fullerene
chemistry continues to be an exciting
field generating many
articles with
promising
new applications every year.
Magnetic nanoparticles
(nanomagnetic
materials)
show great potential for
high-density magnetic storage media.
Recent work
has
shown that C60 dispersed into
ferromagnetic materials such as
iron, cobalt, or
cobalt
iron
alloy can form thin films
with promising magnetic
properties. A number of
organometallic-fullerene
compounds have recently been
synthesized. Of particular
note
9.14
Newer
Synthetic Strategies for
Nanomaterials
are
a ferrocene-like C60 derivative and pair of
fullerenes bridged by a rhodium
cluster.
Some
fullerene derivatives even
exhibit superconducting character.
There has been a
report
of a fullerene containing,
superconducting field-effect device
with a Tc
as high
as
117
K.
5.2
b. Carbon Nanotubes:
Carbon
nanotubes (CNTs) are hollow
cylinders of carbon atoms.
Their appearance is
that
of rolled tubes of graphite such
that their walls are
hexagonal carbon rings and
are
often
formed in large bundles. The
ends of CNTs are domed
structures of six-membered
rings
capped by a five-membered ring.
Generally speaking, there are
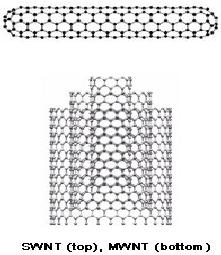
two types of CNTs:
single-walled
carbon nanotubes (SWNTs) and
multi-walled carbon
nanotubes
(MWNTs)
(Fig.10). As their names
imply, SWNTs consist of a
single, cylindrical
graphene
layer, where as MWNTs consist of
multiple graphene layers telescoped
about
one
another. Carbon nanotubes
(CNTs) were first isolated
and characterized by Ijima in
1991.
Since then numerous research
articles have been
published, and new
applications
for
CNTs have been proposed
every year. The unique
physical and chemical properties
of
CNTs, such as structural
rigidity and flexibility continue to
generate considerable
interest.
Additionally, CNTs are
extremely strong, about 100
times stronger
(stress
resistant)
than steel at one-sixth the
weight. CNTs can also act as either
conductors or
semiconductors
depending on their chirality,
possess an intrinsic superconductivity,
are
ideal
thermal conductors, and can also behave as
field emitters.
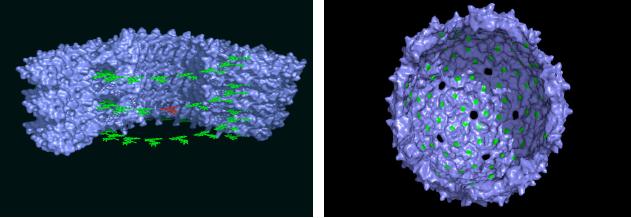
Fig.
9.10. Carbon
nanotubes
Synthetic
Strategies in Chemistry
9.15
Currently,
the physical properties are
still being discovered and disputed.
What makes it
so
difficult is that nanotubes
have a very broad range of electonic,
thermal, and
structural
properties that change depending on
the different kinds of
nanotube (defined
by
its diameter, length, and
chirality, or twist). To make things
more interesting,
besides
having
a single cylindrical wall
(SWNTs), nanotubes can have
multiple walls
(MWNTs)--cylinders
inside the other
cylinders.
(i)
Properties of Carbon
Nanotubes:
With
graphene tubes parallel to the filament
axis,
nanotubes
would inherit several
important properties of `intra-plane'
graphite. This
imparts
a very unique combination of properties
on this material,
namely:
High
aspect ratio structures with
diameters in nanometers, lengths
in
microns
High
mechanical strength (tensile
strength 60GPa) and modulus
(Young's
modulus
1TPa)
--
High electrical conductivity
(10-6 ohm m typically), and for
well
crystallized
nanotubes ballistic transport is
observed
High
thermal conductivity (1750-58 00
W/mK)
--
Being covalently bonded, as electrical
conductors they do not
suffer
from
electro-migration
or
atomic diffusion and thus can
carry high
current
densities (107 -109
A/cm2
)
Single
wall nanotubes can be metallic or
semi-conducting
Chemically
inert, not attacked by strong
acids or alkali
Collectively,
nanotubes can exhibit extremely
high surface area
(ii)
CarbonNnanotubes Developed by Arry Group
Arry
has done a lot of research and
development work in the
carbon nanotubes
synthesis
and
application, and developed a scalable CVD
method to produce high
purity single-
walled
carbon nanotubes (SWNTs) and
multi-walled carbon nanotubes
(MWNTs) with
various
diameters and narrow diameter
distribution. The technology
for producing 5kg
20-40
nm MWNTs per day was evaluated as an advanced
technology in the world
by
CAS
scientists. They can produce
1.5kg SWCNTs per day and
10kg MWNTs with 8-
15nm
in diameter per day. The
production facilities can be enlarged
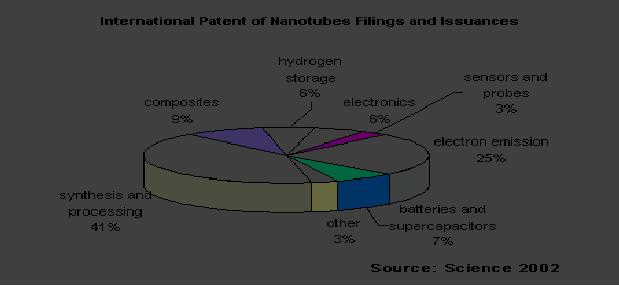
easily. In Fig.11,
the
proportion
of usage as conceived by Array
group is shown.
9.16
Newer
Synthetic Strategies for
Nanomaterials
Fig.
9.11. Illustration given by
Array group [15]
Nanotechnology
develops the basis for
increasingly smaller data
memories with
increasingly
larger storage capacity, for
highly efficient filters for
sewage treatment, for
photovoltaic
windows, for materials which
can be used to build ultra-light engines
and
body
parts in the automobile industry or
for artificial joints which
are better tolerated
by
the
human body due to organic
nano-surfaces. In the future, as
nanoscale molecular self-
assembly
becomes a commercial reality,
nanotech will move into
conventional
manufacturing.
While nanotechnology offers
opportunities for society, it also
involves
profound
social and environmental risks,
not only because it is an
enabling technology
to
the biotech industry, but
also because it involves atomic
manipulation and will make
possible
the fusing of the biological
world with the mechanical
world. There is a
critical
need
to evaluate the social
implications of all nanotechnologies; in
the meantime, the
Arry
Group believes that a
moratorium should be placed on research
involving
molecular
self-assembly and
self-replication.
(iii)
Carbon Nanotube-Based
Nanodevices
Carbon
nanotubes are a hot research
area at the moment. The
excitement has been
fueled
by
experimental breakthroughs that
have led to realistic
possibilities of using
them
commercially.
Applications could include
field emission-based flat panel
displays, novel
semiconducting
devices, chemical sensors, and
ultra-sensitive electromechanical
sensors.
The utility of carbon
nanotubes for molecular
electronics or computers,
first
predicted
by theory and simulations, is now
being explored through
experiments to
Synthetic
Strategies in Chemistry
9.17
fabricate
and conceptualize new devices based on
simulations. Carbon nanotubes
are
now
the top candidate to replace silicon
when current chip features
cannot be made any
smaller
in 10-15 year's time.
Calculations show that
nanotubes can have metallic
or
variable
semiconducting properties with energy
gaps ranging from a few
meV to a few
tenths
of an eV. Experiments probing
the density of states
confirm these
predictions.
Conductivity
measurements on single nanotubes have
shown rectification effects
for
some
nanotubes and ohmic conductance
for others. These properties
suggest that
nanotubes
could lead to a new generation of
electronic devices. Simulations to
investigate
the interaction of water
molecules with a nanotube
tip revealed an
atomistic
understanding
of the interaction, which is
critical in designing commercial-quality
flat
panel
displays around carbon
nanotubes. Their use as
ultra-sensitive electromechanical
sensors
has also been
explored.
5.3
Dendrimers (Organic
Nanoparticles)
In
recent years, a new structural
class of macromolecules, the dendritic
polymers,
has
attracted
the attention of the
scientific community. These nanometer
sized, polymeric
systems
are hyperbranched materials
having compact hydrodynamic
volumes in solution
and
high, surface, functional
group content. They may be
water-soluble but, because
of
their
compact dimensions, they do
not have the usual
rheological thickening
properties
that
many polymers have in
solution. Dendrimers, the
most regular members of
the
class,
are synthesized by step-wise
convergent or divergent methods to
give distinct
stages
or generations. Dendrimers are
defined by their three
components: a central core,
an
interior dendritic structure
(the branches), and an exterior
surface (the end
groups).
Over
50 compositionally different families of
these nanoscale macromolecules,
with
over
200 end-group modifications, have
been reported.They are
characterized by nearly
spherical
structures, nanometer sizes, large
numbers of reactive end
group
functionalities,
shielded interior voids, and
low systemic toxicity. This
unique
combination
of properties makes them
ideal candidates for
nanotechnology applications
in
both biological and materials
sciences. The state of
reports in the current
literature has
been
directed toward their
applications in a broad range of fields,
including materials
engineering,
industrial, pharmaceutical, and
biomedical applications.
Specifically,
nanoscale
catalysts, novel lithographic
materials, rheology modifiers, and
targeted drug
9.18
Newer
Synthetic Strategies for
Nanomaterials
delivery
systems, MRI contrast agents, and bioadhesives
represent some of the
potential
applications.
5.4
Polyhedral Silsesquioxanes
(Inorganic-Organic Hybrid
Nanoparticles)
Hybrid
inorganic-organic composites are an
emerging class of new
materials that
hold
significant promise. Materials
are being designed with
the good physical
properties
of
ceramics and the excellent choice of
functional group chemical
reactivity associated
with
organic chemistry. New
silicon-containing organic polymers, in
general, and
polysilsesquioxanes,
in particular, have generated a great
deal of interest because of
their
potential replacement for and
compatibility with currently
employed, silicon-based
inorganics
in the electronics, photonics, and
other materials technologies.
Hydrolytic
condensation
of trifunctional silanes yields network
polymers or polyhedral
clusters.
Hence
they are known by the
"not quite on the tip of
the tongue" name
silsesquioxanes.
Each
silicon atom is bound to an
average of one and a half (sesqui)
oxygen atoms and to
one
hydrocarbon group. Typical
functional groups that may
be hydrolyzed or condensed
include
alkoxy- or chlorosilanes, silanols, and
silanolates. Synthetic methodologies
that
combine
pH control of hydrolysis/condensation
kinetics, surfactant-mediated
polymer
growth,
and molecular templating mechanisms
have been employed to
control molecular
scale
regularity as well as external
morphology in the resulting
inorganic/organic
hybrids
(from transparent nanocomposites, to
mesoporous networks, to highly
porous
and
periodic organosilica crystallites)
all of which have the
silsesquioxane
stoichiometry.
These inorganic-organic hybrids offer a
unique set of physical,
chemical,
and
size dependent properties that could
not be realized from just
ceramics or organic
polymers
alone. Silsesquioxanes are therefore
depicted as bridging the property
space
between
these two component classes
of materials. Many of these
silsesquioxane hybrid
materials
also exhibit an enhancement in properties
such as solubility, thermal
and
thermomechanical
stability,
mechanical
toughness,
optical
transparency,
gas
permeability,
dielectric constant, and fire
retardancy, to name just a
few.
5.5
Nano-Intermediates
Nanostructured
films, dispersions, high surface
area materials, and
supramolecular
assemblies
are the high utility
intermediates to many products
with improved
properties
such
as solar cells and batteries, sensors,
catalysts, coatings, and drug
delivery systems.
Synthetic
Strategies in Chemistry
9.19
They
have been fabricated using
various techniques. Nanoparticles
are obvious building
blocks
of nanosystems but, require special
techniques such as self-assembly to
properly
align
the nanoparticles. Recent
developments have lead to air
resistant, room
temperature
systems for nanotemplates with features
as small as 67 nm.
More
traditionally,
electron-beam systems are used to
fabricate devices down to 40
nm.
5.6
Nanocomposites
Nanocomposites
are materials with a nanoscale
structure that improve the
macroscopic
properties
of products. Typically, nanocomposites
are clay, polymer or carbon,
or a
combination
of these materials with
nanoparticle building blocks.
Nanocomposites,
materials
with nanoscale separation of phases can
generally be divided into
two types:
multilayer
structures and inorganic/organic composites.
Multilayer structures
are
typically
formed by gas phase
deposition or from the
self-assembly of monolayers.
Inorganic/organic
composites can be formed by sol-gel
techniques, bridging
between
clusters
(as in silsequioxanes), or by coating
nanoparticles, in polymer layers
for
example.
Nanocomposites can greatly enhance the
properties of materials. For
example,
ppm
level impurities can result in
the formation of nanoscale aluminide
secondary
phases
in aluminum alloys, increasing
their strength and corrosion resistance.
Magnetic
multilayered
materials are one of the
most important aspects of nanocomposites
as they
have
led to significant advances in
storage media.
5.6
a. Polymer-Clay Nanocomposites
The
large industrial demand for
polymers has lead to an equally
large interest in
polymer
composites to enhance their properties.
Clay-polymer nanocomposites are
among
the most successful
nanotechnological materials today.
This is because they
can
simultaneously
improve material properties without
significant tradeoffs. Recent
efforts
have
focused upon polymer-layered
silica nanocomposites and other
polymer/clay
composites.
These materials have improved
mechanical properties without
the large
loading
require by traditional particulate
fillers. Increased mechanical stability
in
polymer-clay
nanocomposites also contributes to an increased heat
deflection
temperature.
These composites have a large reduction
gas and liquid permeability
and
solvent
uptake. Traditional polymer composites
often have a marked
reduction in optical
clarity;
however, nanoparticles cause
little scattering in the
optical spectrum and
very
9.20
Newer
Synthetic Strategies for
Nanomaterials
little
UV scattering.Although flame retardant
additives to polymers typically
reduce
their
mechanical properties, polymer-clay
nanocomposites have enhanced barrier
and
mechanical
properties and are less
flammable. Compression-injection molding,
melt-
intercalation,
and coextrusion of the polymer
with ceramic nanopowders can
form
nanocomposites.
Often no solvent or mechanical
shear is needed to
promote
intercalation.
6.
Novel Materials at the
Nanoscale Functional
Nanomaterials
Morphology-controlled
functional nanomaterials have
unique chemical,
mechanical,
electrical,
optical, magnetic or biological
properties that are
distinctly different form
their
macroscopic
analogs
and
provide
new
diverse
opportunities
for
promising
nanotechnologies.
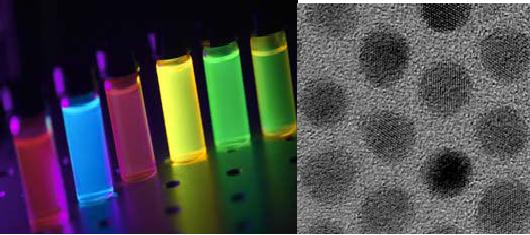
Fig.
9.12 Quantum dots: Colour
tuning [2]
6.1
Nanomaterials Size and
Composition-Tunable Quantum
DotsConducting
Development
of a scalable synthetic strategy is
necessary to address the
quantum dots'
colour-tuning
and stability issues. Alloyed
ZnCdS/Se nanocrystals have
unique
composition-tunable
optical properties including:-High
luminescence/stability -Increased
narrow
luminescence spectral-width. These size and
composition-tunable quantum dots
have
applications in the optoelectronic and
biomedical sectors. (Fig
9.12)
Synthetic
Strategies in Chemistry
9.21
7.
ZnO/TiO2 Nanorods
or Nanoarrays and Silica-Coated
Metal Nanocrystals
Fig.
9.13. Nanoarrays of
ZnO/TiO2 [16]
Researchers
have focused on large-scale growth of
well-aligned ZnO nanorods
on
selected
substrates. Their applications are
short-wavelength optoelectronic devices,
solar
energy
conversion, transparent conducting
coating materials and sensors.
Researchers try
to
find other functional
nanomaterials with diverse
morphologies such as titania
nanorods
and
silica coated nano
crystals.(Fig. 9.13)
8.
Polyhedral Oligomeric Silsesquioxanes
(POSS)
Fig.
9.14. POSS Material
9.22
Newer
Synthetic Strategies for
Nanomaterials
Unique
nanostructured material, hybrid
inorganic and organic compositions at
nanoscale,
development
and characterization of POSS-modified
epoxy and POSS (Fig.
9.14)
containing
high performance polymers
were synthesized to enhance its
thermal and
mechanical
properties.
Advantages:
1.
Numerous potential applications
such as microelectronics, photonics,
aerospace, and
coatings.
2.
POSS materials are found to
be compatible with mostly
all thermoplastics and
thermosets.
3.
POSS materials possess size
controllable, processable and tunable
properties.
8.a
Conducting Polymer Nanofibers
via Electrospinning :
The
major work in this area is
to develop a novel methodology
for synthesising
conducting
polymers with high molecular
weight and excellent solubility. It is
also
necessary
to fabricate conducting polymer
nanofibers via electrospinning
process.
Investigating
size/quantum confinement effect on
the optical, electrochemical
and
conducting
properties of the nanofibers and
finally to explore the possible
applications of
the
conducting polymer nanofibers as
OLED semissive layer, sensors and
molecular
electronics.
CONCLUSION:
The
impact of understanding self-organizing
behavior, and of finding ways to
further
direct
assembly to make exotic nanoscale
properties useful at the macroscale,
clearly will
be
enormous. There are general
rules of controlled synthesis and
directed assembly to be
discovered,
and the systematic application of
these will result in the
addition of many
different
nanostructured materials. Each and every
success in the synthesis
of
nanomaterials
will make available a new subset of
engineering materials, and it is
well
known
from centuries of experience that
the discovery and development of
new synthetic
stratergies
for nanomaterials always
have been the source of new
technology.
REFERENCES:
1.
A.P. Alivisatos, Science, 271
(1996) 993.
2.
A. P. Alivisatos, J.Phys.Chem., 31 (1996)
13226.
3.
A. Henglein., Chem. Rev., 89
(1989) 1861.
Synthetic
Strategies in Chemistry
9.23
4.
M. B. Mohamed, C. Burda, and M. A.
El-Sayed, Nanolett, 1 (2001)
589.
5.
J. H. Fendler, Chem. Mater, 8
(1996) 1616.
6.
H. Gleiter, Acta Mater. 48
(2000) 1.
7.
C. R. Henry, Surf. Sci. Rep.
31 (1998) 231
8.
K.J. Shea, D.A. Loy,
Chem. Mater.13 (2001)
3306.
9.
K.Strawhecker, E. Manias, Chem.
Mater.12 ( 2000)
2943.
10.E.P.
Giannelis Adv. Polym. Sci.
138 (1998) 107.
11.
B. C. Gates, Chem. Rev., 95
(1995) 511.
12.
Thurston et al. Inorg. Chem.
42(6), (2003), 2014.
13.
www.ruf.rice.edu
14.
Quld-Ely et al Chimie 8 (2005)
1906.
15.www.patentstorm.us
16.
www.mrs.org
17.
http://en.wikipedia.org/
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES