 |
MICROEMULSION TECHNIQUES:Significance of Packing Parameter |
| << TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway |
| SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS >> |

7.10
Microemulsion
Techniques
(ii)
Isooctane,
being bulkier with a larger
molecular volume, cannot penetrate
the
surfactant
tails so efficiently, thereby
leading to a more fluid
interface and thus
faster
growth
rates.
Although
these ideas provide
plausible explanations of the
phenomena they remain
controversial.
Measurement
of the surfactant film
rigidities in microemulsions show
that the solvent
type
has only a minor effect.
Solvent molecular volume may
also explain the observed
change
in final particle size. It was
proposed that a more stable
micelle system arises
from
greater
interactions between the
solvent and surfactant tails
which in turn leads to
an
enhanced
ability to stabilize larger
particles. Any increase in rate of
intermicellar exchange
will
result in a higher rate of growth
comparable to nucleation, hence is likely
to generate
systems
with lower polydispersity.
Effect of solvent on the size of
the Ag nanoparticles is
given
in Table 7.4.
Table
7.4. Effect of solvent on
the absorption spectra of
silver nanoparticles synthesized
in
reverse
micelles of AOT (data taken from
ref. 22)
System
Particle
size (nm)
6.0
AOT/decane
22.0
AOT/heptane
5.4
AOT/cyclohexane
Surfactants
and co-surfactants
Various
studies showed that the
choice of surfactant is critical to
the size, shape and
stability of
the
particles. The most commonly
used surfactant is the
anionic AOT, although a variety
of
common
cationic surfactants are also
frequently employed, such as
CTAB or DDAB (di-n-
didodecyldimethylammoniumbromide)
and non-ionics Triton X-100,
polyoxyethylene (5)
nonylphenyl
ether (NP-5) or polyoxyethylene
(9) nonylphenyl ether
(NP-9). For some
systems
co-surfactants
(intermediate chain length
alcohols, such as n-butanol or
n-hexanol) are also
employed.
The
Evolving Synthetic Strategies in
Chemistry
7.11
Among
the anionic surfactants that
form reverse micelles, the
best known are the
systems
derived
from AOT (sodium
1,4-bis-2-ethylhexylsulfosuccinate) in different
non-polar media.
The
reasons are as
follows:
(i)
AOT
has a well-known V-shaped
molecular geometry, giving rise to stable
reverse
micelles
without co-surfactant.
(ii)
AOT
has the remarkable ability
to solubilize water with
values of W (W =
[H2O]/[AOT]) as large as 40-60
depending on the surrounding
non-polar medium, the
solute
and the temperature; however
the droplet size depends
only on the water
amount,
W. The bulk properties of water
(polarity, viscosity, hydrogen
bond ability,
etc.)
either inside the pool
(free) or at the interface
(bound) change with W.
It
is also known that addition of
co-surfactant can reduce the surfactant
concentration in
microemulsion
preparation. Normally, low
molecular weight alcohols,
such as n-butanol can be
used
for this purpose. Their
short hydrophobic chain and
terminal hydroxyl group is
known to
enhance
the interaction with
surfactant monolayers at the
interface, which can influence
the
curvature
of the interface and internal
energy. The amphiphilic
nature of co-surfactants
could
also
enable them to distribute between
the aqueous and oil
phase.
In
general, it was concluded that
the addition of a co-surfactant
leads to a higher fluidity
of
the
interfacial film, thus
increasing the rate of intermicellar
exchange (but also leading to
a
higher
curvature of the droplets), so
smaller particles.
Surfactant
Concentration
When
the amount of water and oil
is kept at fixed values, an
increase of the amount of
surfactant
will
increase the number of
droplets. It means that the
number of metal ions per
droplet will
decrease
and consequently the size of the
particles. The morphology of reverse
micelles is
different
with the surfactant
concentration. At different
concentrations, the surfactant
molecules
can
form various molecule aggregations
(Fig. 7.2).
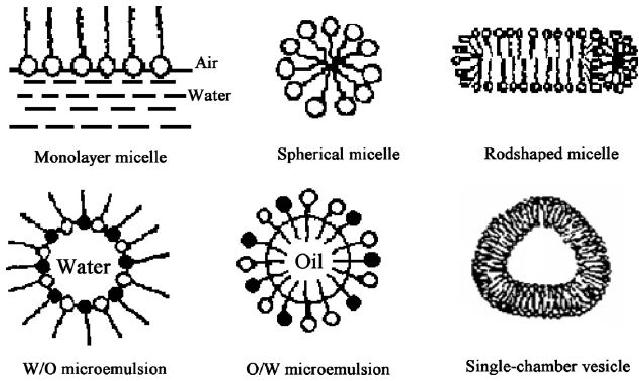
7.12
Microemulsion
Techniques
Fig.
7.2. Structures of different
micelles (reproduced from
ref. 26)
It
was found that the change of
micelle structures had an energetic
barrier. The main points
are
given
as follows:
(i)
At
a low surfactant concentration,
only spherical micelles
appear in solution.
(ii)
When
the surfactant concentration in
the solution reaches a
well-defined saturation
value,
i.e., second critical
micelle concentration (second CMC),
the energetic barrier
will
be overcome and the micelle
structure will change from a spherical
micelle to
other
special structure, and then the
micelle will be steady again at a
new
concentration
range. For example, if the
concentration of surfactant attains to
40
50%,
the spherical micelle turns
into rod-shaped or column-shaped
micelle.
Furthermore,
the micelle is also able to self-assemble
into layer or liquid
crystal
structure.
The
special micelles formed at different
concentrations of surfactants can be
usually used as
effective
structure-directing agents to prepare
nanoparticles with desired
morphologies. Thus,
the
micelle
formed by the surfactant
with a proper concentration can
offer an appropriate growth
condition
for nanoparticles.
The
Evolving Synthetic Strategies in
Chemistry
7.13
Nature
of the Precipitating Agent
(reducing agent)
The
main point that should be
followed in the selection of
suitable reducing agent in
the
preparation
of nanoparticles is it must be stable in an
aqueous environment and does
not react
with
the other components of the
reverse micelle system. As a general
rule, a fast
nucleation
process
will result in the production of
small particles.
In
most of the cases, water
soluble sodium borohydride and
hydrazine (usually N2H4.2HCl
or
N2H4.H2O)
are used as effective
reducing agents. Eventhough bubbled
H2 gas results in
the
reduction
of metal particles, the
kinetics is not desirable, particularly
at room temperature. As it
was
mentioned earlier, faster
exchange between the reactants and
fast nucleation process
will
give
smaller particles. So both
sodium borohydride
(NaBH4)
and hydrazine are efficient
reducing
agents
for most of the transition
metal salts. The reduction
process is in this case
completed
instantly
and is very fast in comparison to
pure hydrogen.
When
increasing the concentration of
hydrazine while the
concentration of metal salt is
kept
constant,
a decrease in the particle size is
observed. This was shown when Ni
particles were
prepared
in a microemulsion containing
cetyltriammonium bromide (CTAB) as
surfactant, n-
hexanol
as oil phase, water as
aqueous phase and hydrazine as
reducing agent at a temperature of
350
K (Capek, 2004). The diameter of
the nickel particles
decreases when the ratio of
the
hydrazine
to nickel chloride concentrations
increases. The diameter of
the particles reaches
a
constant
value when this ratio is
above 10.
Effect
of the Micellar
Template
It
was claimed that particle
shape can be controlled by using
micellar templates. A
simple
surfactant/water/oil
system can produce many
different self-assembly structures: by
changing
composition,
one can obtain spheres (reverse
micelles or micelles), cylinders,
interconnected
cylinders
and planes, termed lamellar phases,
which also can re-organise into
onion-type
structures.
Hence in theory many possible
nanoparticle structures could be
grown inside these
different
shaped templates. Despite plenty of
work, there is still much
controversial debate on
these
aspects.
Influence
of ion/molecular adsorption
Anion
species added as electrolyte is
important for generating
different shapes of
metal
nanocrystals.
The initial micellar shape
is shown to be largely unaffected by
these additives. For
example,
in the case of copper nanoparticle
systems, a large excess of hydrazine
favours disk
7.14
Microemulsion
Techniques
over
spherical particles. In both
cases, Pileni et
al.
(2003) postulate that
selective adsorption of
molecules
or ions on to facets of the nanocrystal
affects growth in certain
directions, explaining
the
apparent preference for certain
shapes.
It
was also known that pH affects
the shape of nanocrystals,
for example,
nanostructured
NiZn
ferrites (Uskokovic et
al.,
2005). When the pH is lower,
needle-like nanocrystals
are
formed,
whereas other spheres are observed at
higher pH. One possible
reason for this is due
to
an
increased number of hydroxyl
ions at higher pH which
eliminate the sulphate and
bromide
ions,
hampering their ability to
promote uniaxial
growth.
SUMMARY
The
most remarkable features of
the microemulsion technique are:
(i) particle size and
composition
can be controlled to a great extent and a
narrow particle size distribution can
be
obtained
and (ii) Bimetallic particles can be
obtained at room
temperature.
A
large number of different
nano-materials can be synthesised in water-in-oil
microemulsions
and
reverse micelles. Particle growth
has shown to be strongly dependent on
intermicellar
exchange
rates. The resultant
particle size appears to be dependent on
dominant parameters,
namely
solvent type, surfactant/co-surfactant
type, concentration of the
reagents and composition
via
[water]:[surfactant] ratio, W.
However,
the generality of the
factors that affects the
shape of particle remains to be
established.
REFERENCES
1.
K. Tapas and A. Maitra, Adv.
Colloid Interface Sci.,
59 (1995) 95.
2.
J.J. Silber, U.A. Biasuttia,
E. Abuinb and E. Lissi, Adv.
Colloid Interface Sci.,
82 (1999)
189.
3.
T. Tadros, P. Izquierdo, J. Esquena and
C. Solans, Adv.
Colloid Interface Sci.,
108-109
(2004)
303.
4.
S. Eriksson, Ulf Nyl�n, S. Rojas and M.
Boutonnet,
Applied
Catalysis A: General 265
(2004)
207.
5.
T.P. Hoar and J.H. Schulman,
Nature
152
(1943) 102.
6.
M.-P. Pileni, Nature
2
(2003)145.
7.
T. Tago, T. Hatsuta, K. Miyajima, M.
Kishida, S. Tashiro and K. Wakabayashi,
J.
Am.
Ceram.
Soc. 85
(2002) 2188.
The
Evolving Synthetic Strategies in
Chemistry
7.15
8.
S. Rojas, F.J. Garcia-Garcia, S.
Jaras, M.V. Martinez-Huerta, J.L.
Garcia Fierro and M.
Boutonnet,
Applied
Catalysis A: General 285
(2005) 24.
9.
M. Boutonnet, J. Kitzling and P. Stenius,
Colloids.
Surf. 5
(1982) 209.
10.
A.P. Herrera, O. Resto, J.G.
Briano and C. Rinaldi, Nanotechnology
16
(2005) S618.
11.
L. Xiong and A. Manthiram, Solid
State Ionics 176
(2005) 385.
12.
V. Raghuveer, P.J. Ferreira, A.
Manthiram, Electrochem.
Commun. 8
(2006) 807.
13.
P.S. Khiew, S. Radiman, N.M.
Huang, Md Soot Ahmad and K. Nadarajah,
Mater.
Lett.
59
(2005) 989.
14.
J. Eastoe and A.R. Cox, Colloids
Surf A Physicochem. Eng.
Asp. 101
(1995) 63.
15.
Ch. Venkateswara Rao and B.
Viswanathan,
J.
Phys. Chem. C 111
(2007) 16538.
16.
V. Raghuveer, Keshav, Kumar and
B.Viswanathan, Indian
J. Eng. Mater. Sci. 9
(2002)
137.
17.
A. Bumajdad, M.I. Zaki, J. Eastoe and L.
Pasupulety, Langmuir
20
(2004)11223.
18.
S. Xing, Y. Chu, X. Sui and Z.
Wu, J.
Mater. Sci. 40
(2005) 215.
19.
E. Carpenter, J.A. Sims,
J.A. Wienmann, W.L. Zhou and
C.J. O'Connor, J.
Appl. Phys.
87
(2000) 5615.
20.
R. Schom�cker, M.-J. Schwuger and K.
Stickdorn, Chem.
Rev. 95
(1995) 849.
21.
V. Uskokovic and M. Drofenik, Colloids
Surf. A Physicochem. Eng.
Asp. 266
(2005)168.
22.
R.P. Bagwe and K.C. Khilar,
Langmuir
16
(2000) 905.
23.
C. Petit, P. Lixon and M.P.
Pileni, J.
Phys. Chem. 97
(1993) 12974.
24.
I. Capek, Adv.
Colloid Interface Sci.,
110 (2004) 49.
25.
Taleb, C. Petit and M.P.
Pileni, Chem.
Mater. 9
(1997) 950.
26.
J.H. Fendler, Chem.
Rev. 87
(1987) 877.
27.
W.L. Zhou, E.E. Carpenter,
J. Sims, A. Kumbhar and C.J. O'Connor,
Mater.
Res. Soc.
Symp.
Proc. 581
(2000) 107.
28.
C.-H. Lu and H.-C. Wang, J. Mater.
Chem. 13
(2003) 428.
29.
C. Liu, A.J. Rondinone and
Z.J. Zhang, Pure
Appl. Chem. 72
(2000) 37.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES