 |
SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE |
| << SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE |
| TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway >> |
Chapter
- 5
SOL-GEL
TECHNIQUES
L.
Hima Kumar
INTRODUCTION
Sol-gel
processing generally refers to the
hydrolysis and condensation of
alkoxide-based
precursors.
It can produce ceramic and
glasses with better purity
and homogeneity than
high
temperature
conventional process. Sol-gel
process can be used to
produce a wide range
of
oxides
in various forms, including
powders, fibers, coatings
and thin films, monoliths
and
porous
membranes. Organic/inorganic hybrids can
also be made, where a gel, usually
silica,
is
impregnated with polymer or
organic dyes with specific
properties. The advantage of
sol-
gel
method is, metal oxides
which are difficult to attain by
conventional methods can be
produced
by using the sol-gel
process. Another benefit is
that the mixing level of
the solution
is
retained in the final
product, often in the
molecular scale.
Sol-gel
processing proved useful in the
manufacturing of stained glass
and for the
preparation
of oxide materials from
sol-gel precursors. Sol-gel derived
products have
numerous
applications. One of the
promising application areas is
for coatings and thin
films
used
in electronics, optical and
electro-optical components and
devices, such as
substrates,
capacitors,
memory devices, infrared
(IR) detectors and wave
guides. Antireflection
coatings
are
also used for automotive and
architectural applications. Submicron
particle size powders
of
single and multicomponent
composition can be made for
structural, electronic, dental,
and
biomedical
applications. Composite powders
can also be used as
agrochemicals or herbicides.
Optical
and refractory fibers are
used for fiber optics
sensors and thermal
insulation,
respectively.
In addition, sol-gel techniques
can be used to infiltrate
fiber performs to
make
composites.
Glass monoliths and coating
and organic/inorganic hybrids are
under
development
for lenses, mirror
substrates, graded index
optics, optical filters,
chemical
sensors,
passive and nonlinear active
waveguides, and lasers.
Membranes for separation
and
filtration
processes also are being
investigated, as well as catalysts.
Biomolecules (such as
proteins,
enzymes, antibodies, etc.) are
incorporated into sol gel
matrices, which can be
used
for
the monitoring of biochemical
processes, environmental testing,
food processing, and
drug
delivery for medicine or
agriculture.
Sol-gel
process, starting with a
metal alkoxide solution and
going through various
processes
and operations until a final
product is obtained, such as a
dense film, an aerogel,
a
ceramic
fiber was shown in Fig.
5.1.
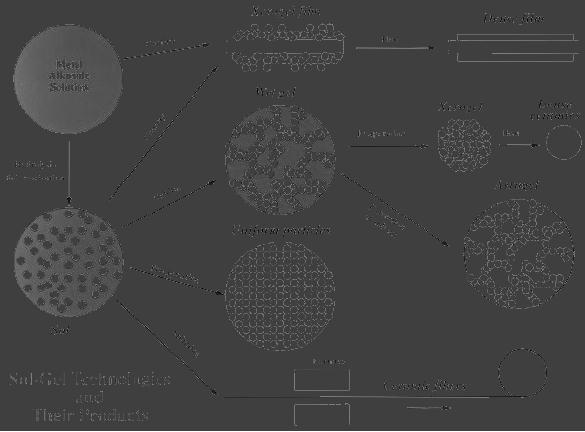
5.2
Sol-Gel
Methods
Fig.
5.1
Schematic
representation
of
the
sol-gel
process
(reproduced
from
http://www.chemat.com/assets/images/Flowchat72.jpg)
DEFINITIONS
A
colloid
is a
suspension in which the dispersed phase
is so small (~1-1000 nm)
that the
gravitational
forces are negligible and
interactions are dominated by
short-range forces,
such
as
van der Waals attraction
and surface charges.
A
sol
is a
colloidal suspension of solid particles
in a liquid.
An
aerosol
is a
colloidal suspension of particles in a
gas.
A
gel
consists
of a three dimensional continuous
network, which encloses a
liquid phase, in a
colloidal
gel, the network is built
from agglomeration of colloidal
particles and is limited
by
the
size of container. In a polymer
gel the particles have a
polymeric sub-structure made by
aggregates
of sub-colloidal particles. Generally,
the sol particles may
interact by van der
Waals
forces or hydrogen bonds. A gel
may also be formed from
linking polymer
chains.
SOL-GEL
SYNTHESIS
Sol-gel
synthesis is a particular approach to the
preparation of glasses and
ceramics at low
temperatures,
which may precede either by
the metallorganic
route,
with metal alkoxides
in
Synthetic
Strategies in Chemistry
5.3
organic
solvents, or by the inorganic
route,
with metal salts (chlorides,
oxychlorides, nitrates,
etc)
in aqueous solution.
GENERAL
MECHANISM
Disregarding
the nature of the precursors,
the sol-gel process can be
characterized by a series
of
distinct steps.
Step
1: Formation of the `sol'
i.e. stable solutions of the
alkoxide or solvated metal
precursor.
Fig.
5.2. Formation of `sol'
(reproduced form ref.
3)
Step
2: Gelation resulting from
the formation of an oxide- or
alcohol-bridged network
(the
gel) by a
polycondensation or polyesterification
reaction that results in a dramatic
increase in
the
viscosity of the solution. If so
desired, the gel may be
cast into a mold during
this step.
Step
3: Aging of the gel, during
which the polycondensation
reactions continue until the
gel
transforms
into a solid mass,
accompanied by contraction of the
gel network and expulsion
of
solvent
from the gel pores. Ostwald
ripening and phase
transformations may
occur
concurrently
with syneresis. The aging
process of gels can exceed 7 days
and is critical to
the
prevention
of cracks in gels that have been
cast.
Step
4: Drying of the gel is,
loss of water, alcohol and
other volatile components,
first as
syneresis
(expulsion of the liquid as
the gel shrinks), then as
evaporation of liquid from
with
in
the pore structure with
associated development of capillary
stress which frequently leads
to
cracking.
This also includes super critical
drying, in which capillary
stress is avoided by
the
use
of supercritical fluids, such as
CO2, in
conditions where there are
no liquid/vapor
densities.
Drying process is complicated
due to fundamental changes in
the structure of the
gel.
The drying process has
itself been broken into four
distinct steps:
5.4
Sol-Gel
Methods
(i)
Constant rate period, (ii)
the critical point, (iii)
the first falling rate
period, and (iv)
the
second
falling rate period. If
isolated by thermal evaporation,
the resulting monolith is
termed
a
xerogel. If the
solvent is extracted under
supercritical or near supercritical
conditions, the
product
is an aerogel.
Fig.
5.3. Formation of gel
(reproduced form ref.
3)
Fig.
5.4. Aging of gel
(reproduced form ref.
3)
Synthetic
Strategies in Chemistry
5.5
Step
5: Dehydration, during which
surface-bound M-OH groups are removed,
thereby
stabilizing
the gel against rehydration.
This is normally achieved by
calcining the monolith
at
temperatures
up to 800 �C.
Step
6: Densification and decomposition of
the gels at high temperatures (T
>
800 �C). The
pores
of the gel network are
collapsed, and remaining
organic species are volatilized.
This
step
is normally reserved for the
preparation of dense ceramics or
glasses.
Fig.
5.5. Densification (reproduced
form ref. 3)
INORGANIC
ROUTE
The
growth of metal-oxo polymers in a
solvent inorganic polymerization
reactions through
hydrolysis
and condensation of metal
alkoxides M(OR)Z,
where M = Si, Ti, Zr, Al, Sn,
Ce,
OR
is an alkoxy group and Z is
the valence or the oxidation
state of the metal, occur in
two
steps
First
step: hydrolysis, Metal cations,
Mz+,
are formed by dissolving
metal salts in water,
are
solvated
by water molecules as
follows:
Mz+ + hH2O
[M(OH2)h]z+
---------------------------------------------------------(1)
where
h
is
the coordination number of
the cation. The
electron-transfer weakens the
O-H
interactions
of bound water, and
depending on the pH of the
solution, various degrees
of
hydrolysis
(deprotonation) can be
induced:
[M(OH2)]z+
[M-OH](z-1)+
+ H+
[M=O](z-2)+
+ 2H+ ------------------------------(2)
In
general, hydrolysis reaction is
facilitated by increase in the charge
density on the metal,
the
number
of metal ions bridged by a
hydroxo or oxo ligand, and
the number of
hydrogens
contained
in the ligand. It also
affected by the pH. The
typical effects of charge and pH
are
shown
in Fig. 5.6, where three
domains corresponding to aquo,
hrdroxy and oxo ions
are

5.6
Sol-Gel
Methods
defined.
Acidic conditions force the
equilibria (Eq. 2) to the
left and favor the
formation of
hydroxo
ligands, whereas basic conditions will
force the equilibria to the
right and favor
oxo
ligands.
The charge on the metal,
however, also plays a
significant role in the
above
equilibria
with respect to pH, given
that highly positive charges
on the metals tend to
substantially
weaken the O-H bonds
and favor the formation of
oxo ligands.
Fig.
5.6. Relationship between charge, pH
and hydrolysis equilibrium of
cations (reproduced
form
ref. 1)
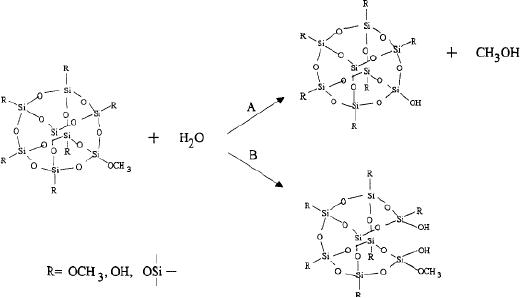
Second
step: polycondensation process leading to
the formation of branched
oligomers and
polymers
with a metal-oxo based
skeleton and reactive
residual hydroxo and alkoxy
groups
M-OH
+ XO-M
M-O-M
+ XOH
--------------------------------------------------------(3)
This
condensation step occurs by
either of the following
ways
1.
Olation, is
a condensation in which a hydroxyl
bridge is formed.
2.
Oxolation,
is condensation in which a oxo
(-O-) bridge is
formed.
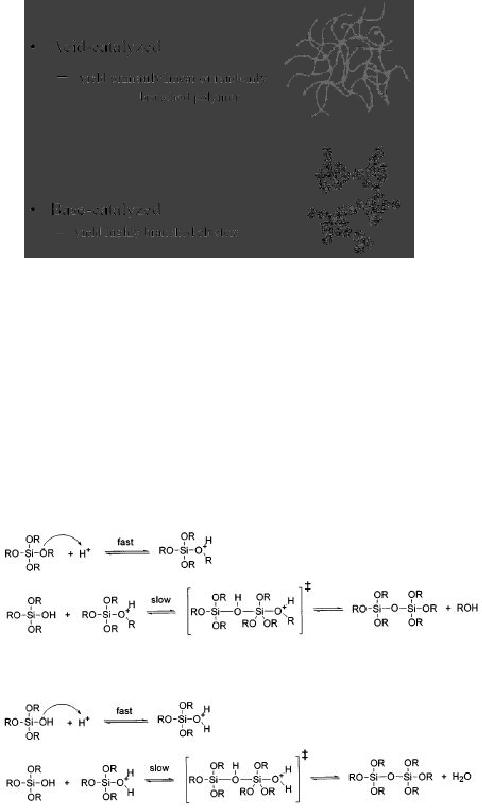
Synthetic
Strategies in Chemistry
5.7
Chemical
Reactivity of Metal
Alkoxides
The
chemical reactivity of metal
alkoxides can be examined
under acidic and
basic
conditions,
as shown in Fig. 5.7.
Fig.
5.7. Polymer structures formed in
acid or basic environments (reproduced
from ref. 1)
As
with initial hydrolysis,
condensation reactions may be
either acid catalyzed or
base
catalyzed
and either cases the
reaction proceeds via a
rapid formation of charged
intermediate
by
a reaction with a proton or
hydroxide ion, followed by
slow attack of second
neutral
silicon
species.
Under
acidic conditions (e.g. with
mineral acids), hydrolysis is faster
than condensation, as
shown
in the following
reaction:
or
5.8
Sol-Gel
Methods
For
Si(OR)4-n(OH)n, under
acidic conditions (pH <
4), the rate of hydrolysis
will always be
faster
than the rate of
condensation due to the
ability of -OR groups to
better stabilize the
transition
states. Condensation involves
the attack of silicon atoms
carrying protonated
silanol
species on neutral Si-OH
nucleophiles. A bushy network of
weakly branched
polymers
is obtained under acidic
conditions.
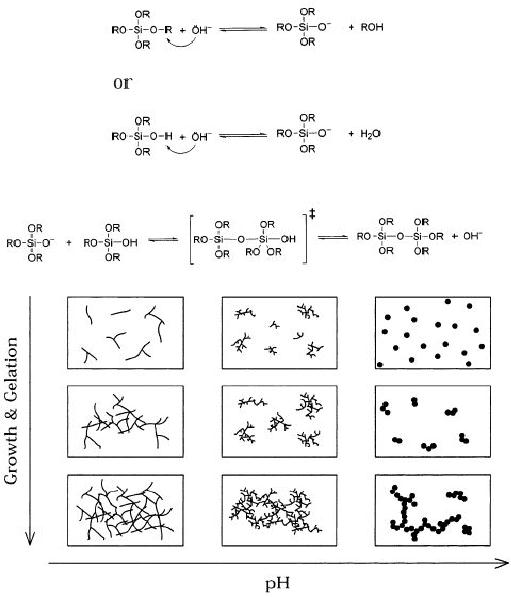
followed
by
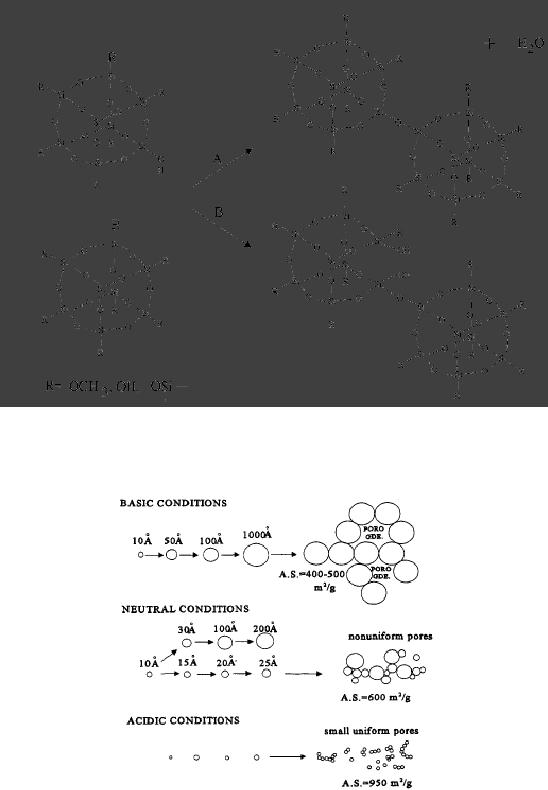
Fig.
5.8. Effect of pH on particle
morphology in sol-gel reactions
(reproduced from ref.
6)
The
result of basic catalysis is an
aggregation (monomer cluster)
that leads to more compact
highly
branched silica networks
that are not interpenetrable
before drying and thus
behave as
Synthetic
Strategies in Chemistry
5.9
discrete
species. The differences
between acid and base
catalyzed reactions and
the
consequences
for particle morphology are
conceptually represented in Fig.
5.8.
Gelation
Gelation
is freezing in a particular structure
(i.e. may be considered as a
rapid solidification
process).
Gelation occurs when links
form between silica sol
particles produced by
hydrolysis
and
condensation, to such an extent
that a giant spanning
cluster reaches across
the
containing
vessel. In other words, as the
sol particles grow and
collide, lead to the
condensation
and then forming
macroparticles. The sol
becomes a gel when it can
support a
stress
elastically. This is typically
defined as the gelation
point or gelation time.
All
subsequent
stages of processing depend on the
initial structure of the wet
gel formed in the
reaction
bath during gelation. Under
acid catalysis polymeric gels
are gradually formed,
as
depicted
in Fig. 5.8. Under basic
conditions and/or with
higher additions of water,
more
highly
branched clusters are formed,
which behave as discrete
species. If the total
concentration
of alkoxysilane is low, e.g., <
~0.3 M, gelation leads to formation of
colloidal
silica
(Stoeber process).
Aging
When
a gel is maintained in its
pore liquid, its structure
and properties continue to
change
long
after the gel point.
This process is called
aging. During aging, four
processes can occur,
singly
or simultaneously, including
polycondensation, syneresis, coarsening,
and phase
transformation.
Polycondensation
reactions occur continuously,
within the gel network as
long as
neighboring
silanols are close enough to react.
This increases the
connectivity of the
network
and
its fractal dimension.
Usually in alkoxide-based gels,
the chemical hydrolysis
reaction is
rapid,
especially, when the sol is
acid catalyzed, and is
completed in the early
stages of sol
preparation.
Since the chemical reaction
is faster at higher temperatures, aging
can be
accelerated
by hydrothermal treatment, which
increases the rate of the
condensation reaction.
Syneresis
is the spontaneous shrinkage of the
gel and resulting expulsion
of liquid from
the
pores. Syneresis in alcoholic gel systems
is generally attributed to formation of
new
bonds
through condensation reactions,
which increases the bridging
bonds and causes
contraction
of the gel network. In
aqueous gel systems or
colloidal gels, the
structure is
controlled
by the balance between
electrostatic repulsion and
attractive van der Waals
forces.
Therefore,
the extent of shrinkage is
controlled by additions of
electrolyte.
5.10
Sol-Gel
Methods
Coarsening
or Ostwald ripening is the
irreversible decrease in surface area
through
dissolution
and reprecipitation processes.
Structural changes attributed
primarily to surface
energy
effects. It is well known
that surfaces exhibiting
positive radii of curvature
dissolve
more
readily than surfaces
exhibiting negative radii of
curvature. If a gel is immersed in
a
liquid
in which it is soluble, dissolved
material will tend to precipitate
into regions of
negative
curvature. Therefore, as the
dissolution rate increases, dissolution
redeposition
results
in neck formation, causing
the gel structure to become
fibrillar and the pore
formation.
Further,
when dissolution is extensive,
the gel network would
break down and ripen to
form a
colloidal
sol.
During
aging, there are changes in
most physical properties of the
gel. Time,
temperature,
solvent
and pH conditions affect the
aging process and lead to
structural modifications.
Heating
the gel in water at 80-100
�C
can strengthen the weak
gel structure and
generally
brings
about reinforcement, but
does not modify the
pore structure. The pH of
the wash water
during
the washing of pore liquor
out of gel is critical in
the case of gels made from
acid-
catalyzed
silicate precursors. The final
properties of such gels depend on
both the pH at
which
the gel was formed
and the pH at which it was
aged before drying.
Drying
Drying
is nothing but removing of
the solvent phase. The
method is influenced by
the
intended
use of the dried material.
If powdered ceramics are desired, no
special care need be
exercised
to prevent fragmentation; if monoliths
from colloidal gels are desired,
the drying
procedures
are largely determined by
the need to minimize internal
stresses associated
with
the
volume changes on drying and
the capillary forces in the
gel pores.
There
are three stages of
drying:
During
the first stage of drying,
the volume change of the gel
equals the volume of
�
evaporated
liquid. The gel network is
still flexible and can
rearrange to accommodate the
decreasing
volume. The compliant gel
network is deformed by the
large capillary
forces,
which
causes shrinkage of the
object. All pores are filled
with solvent and no
liquid-air
interfaces
are present. In classical large-pore systems,
this first stage of drying
is called "the
constant
rate period" because the
evaporation rate per unit
area of the drying surface
is
independent
of time. First stage of
drying ends when shrinkage
stops.
Second
stage of drying begins when
the "critical point" is
reached (Fig .8). The
packing
�
density
of the solid phase increases
with increasing the strength
of the network, and
resists
further
shrinkage. This stage is
called critical point. As
drying proceeds, the gel
network
Synthetic
Strategies in Chemistry
5.11
becomes
more restricted and the
removal of liquid leads to the
formation of such
interfaces
and
the development of capillary
stresses. The liquid flows
to the surface where
evaporation
takes
place. The flow is driven by
the gradient in capillary
stress. Because the rate
of
evaporation
decreases in second stage,
classically this is termed
"the first falling rate
period'.
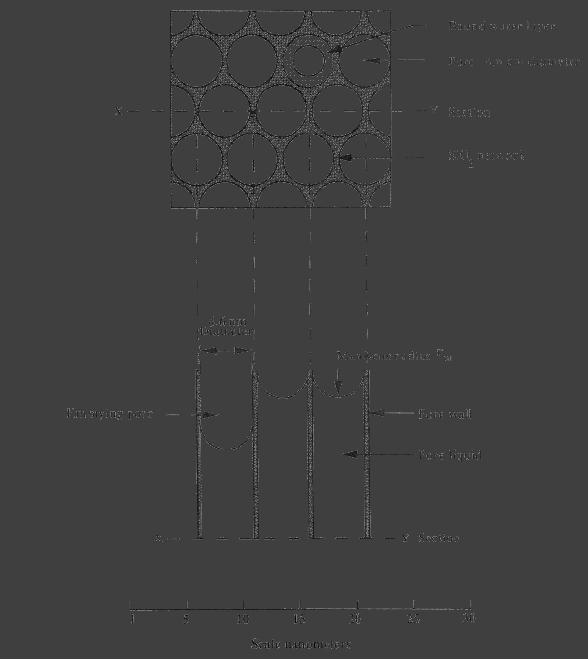
Fig.
5.9. Schematic representation of
gel surface at the end of
first stage (critical
point)
(reproduced from ref.
4)
When
the pores have substantially
emptied, surface films along
the pores cannot be
�
sustained.
This is third stage of
drying, which is also called
as "second falling rate
period".
The
remaining liquid can escape
only by evaporation from
within the pores and
diffusion of
vapor
to the surface. During this
stage, there are no further
dimensional changes but just
a
5.12
Sol-Gel
Methods
slow
progressive loss of weight
until equilibrium is reached, determined
by the ambient
temperature
and partial pressure of
water.
The
capillary pressure, P, developed in a
cylindrical capillary of radius r,
partially filled
�
with
a liquid of wetting angle
α,
can be expressed by:
2γ
cos
α
ĆP =
r
where
γ
is
the surface tension. When
evaporation leads to the formation of
menisci, the
different
radii of the pores cause
unequal capillary pressures to generate
differential stresses.
If
the stress difference
locally exceeds the strength
of the gel network, a crack
will result
(drying
failure). When the gel
has had insufficient aging
and strength, does not
possess the
dimensional
stability to withstand the
increasing compressive stress and leads
to cracking in
the
first stage. Most failure of
drying occurs during the
early part of second stage,
the point at
which
the gel stops shrinking.
This is the point at which
the meniscus falls below
the surface.
Generally,
fractures associated with excessive
capillary forces can be eliminated
or
reduced
by one or more of the
following proceedings:
strengthening
the gel by
reinforcement
�
enlarging
the pores
�
reducing
the surface tension of the
liquid with surfactants
�
making
the interior surfaces
hydrophobic
�
evacuating
the solvent by
freeze-drying
�
Operating
under hypercritical
conditions.
�
Xerogel
and Aerogel Formation
As
a gel is evaporated to dryness either by
thermal evaporation or supercritical
solvent
extraction,
the gel structure changes in
some cases substantially.
During thermal drying
or
room-temperature
evaporation, capillary forces induce
stresses on the gel that
increases the
coordination
numbers of the particles and
induces collapse of the
network. The increase in
particle
coordination numbers results in the
formation of additional linkages
that strengthen
the
structure against further
collapse and eventually leads to
the formation of a rigid
pore
structure.
The structure of the
resulting xerogel can
therefore be considered a
collapsed,
highly
distorted form of the
original gel network.
The
supercritical extraction of solvent
from a gel does not
induce capillary stresses
due to
the
lack of solvent-vapor interfaces. As a
result, the compressive forces exerted on
the gel
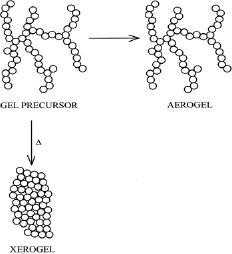
Synthetic
Strategies in Chemistry
5.13
network
are significantly diminished
relative to those created during
formation of a xerogel.
Aerogels
consequently retain a stronger
resemblance to their original gel
network structure
compared
to xerogels (Fig.
5.10).
Fig.
5.10. Structural relationship
between a sol-gel precursor, a
xerogel, and an
aerogel
(reproduced
from ref. 6)
Densification
Densification
is the last treatment
process of gels during which
the dried gel is heated
to
convert
into a dense ceramic. The
sintering of gels, where the
pore network is collapsed
and
organic
species are volatilized, is a
critical factor in determining
the size and morphology
of
the
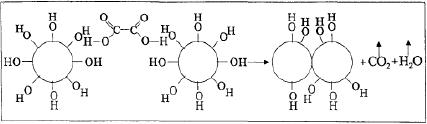
sol-gel product. For silica
gels, the following
reactions occur during the
densification:
desorption
of physically adsorbed solvent and
water from the walls of
micropores (100-
�
200
�C)
decomposition
of residual organic groups
into CO2 + H2O
(300- 500 �C)
�
collapse
of small pores (400-500
�C)
�
collapse
of larger pores (700-900
�C)
�
continued
polycondensation (100-700
�C)
�
If
powdered ceramics are
desired, no need to take care to
prevent fragmentation.
Attention
must,
however, be directed to the
removal of organics to avoid
undesirable bloating,
foaming
or
blackening. Where as, for
monoliths preparation, special
care must be taken to
ensure
complete
removal of water, organic
groups or decomposition products,
prior to micropore
collapse
to avoid the development of
stresses leading to fragmentation
for the production of
large
monoliths of SiO2.
5.14
Sol-Gel
Methods
Sintering
and densification phenomena also
take place, via typical
sintering mechanisms
such
as
evaporation condensation, surface
diffusion, and grain
boundary and bulk diffusion.
The
small
particle size of the powders
lead to high reactivities
and enhanced sintering
and/or
coarsening
rates (the principal process
involved in densification is often
viscous sintering).
As
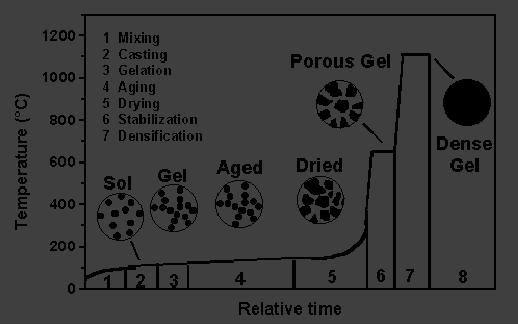
illustrated in Fig. 5.11,
densification of a gel network
occurs between 1000 and
1700 �C
depending
upon the radii of the pores
and the surface area. Fig.
5.11, describes the
overall
development
of a sol-gel process for
glass in relative time and
temperature scales.
This
schematic
representation summarizes from
establishment of a sol condition to
formation of a
dense
material.
Fig.
5.11. Time vs. Temperature
dependence in a sol-gel process
(reproduced
from ref. 4)
APPLICATIONS
Monoliths
The
physical properties of gel-derived
glasses are usually closely
similar to those of
glasses
obtained from the melt.
Gel-derived powders are used
as batch ingredients
for
glass
melting primarily because of
the high degree of chemical
homogeneity they
offer.
This
leads to shorter melting times
and lower melting
temperature as well as
compositional
uniformity. The key to glass
formation is the development of
an
appropriate
heat treatment schedule to remove the
residual organic groups and
achieve
pore
collapse without inducing
crystallization in the sample. The most
attractive feature is
the
development of novel glass
compositions: CaO-SiO2 or Na2O-ZrO2-SiO2 with high
ZrO2 content, which is
impossible to obtain from
the melt, because the
cooling rate must
Synthetic
Strategies in Chemistry
5.15
be
high to avoid detectable
crystallization. Monoliths are
defined as bulk gels cast
to
shape
and processed without
cracking. Monolithic gels are
potentially interesting
because
complex
shapes may be formed at room
temperature and consolidated at
rather low
temperatures
with out melting. The
principle applications of monoliths
are optical ones:
fiber
optic preforms, lenses and
other near-net-shape optical components,
graded
refractive
index glasses and
transparent foams (Aerogels) used as
Cherenkov detectors
and
as super insulation.
Fibers
Fibers
can be drawn directly from
polymer sols by controlling
the viscosity of acid
catalyzed
alkoxide
gels. A key to prevent premature
gelation is to maintain water at a
lower level. High
viscosity
stabilizes the fibers
against spheroidization during
the drawing operation.
Both
silica
and titania fibers have been
obtained at near room
temperature, but without
sintering,
they
remain porous and contain
some unreacted alkoxide. The
porosity decreases
with
increasing
temperature. Sol-gel method
using metal alkoxide as precursor
has been applied to
the
preparation of oxide glass
fibers other than
SiO2.
Fibers produced by sol-gel
processing
are
an interesting application for
the manufacture of optical
waveguides (A device
that
constrains
or guides the propagation of
electromagnetic radiation along a
path defined by the
physical
construction of the guide is
called waveguide). The
dispersion-casting technique
produces
silica fibers with optical
loses of about 3.5
dB/km.
Thin
films and coatings
Thin
films and coatings were
the first commercial
application of sol-gel processing
technology.
Films are applied either by
dip coating or spin coating
techniques, using
polymeric
alkoxide sols. By controlling the
precursor chemistry and
deposition conditions,
pore
size, porosity, surface area, and
refractive index of the
films can be controlled.
Large
substrates
may be accommodated and it is possible to
uniformly coat both sides of
planar and
axially
symmetric substrates such as
pipes, tubes, rods and
fibers.
Generally,
ceramics are more resistant
than metals to oxidation, corrosion,
erosion and
wear.
Moreover, they can have
good thermal and electrical
properties that make
them
particularly
interesting as coating materials.
Ceramic coatings are usually
deposited on metals
for
improving their performances in
high temperature aggressive environments.
Some
important
applications, include improving
resistance against gas,
solid, condensed, and
molten-phase
corrosion, to localized overheating
and melting; decreasing fretting
and wear;
decreasing
heat losses and/or reflecting
radiations in high temperature
systems.
5.16
Sol-Gel
Methods
A
major development was the
formation of multiple transparent
layers that produce a
gradual
change
in refractive index from the
air interface to that of the
substrate. Antireflection
coatings
are also a vast application of
the sol-gel technique, for
example, in store front
windows,
to allow light transmission
but reduced glare. Such coatings
are also used in
solar
cells
and laser optics. A head up display is a
unique application of these
coatings: by
controlling
the reflectance to transmission
ratio, the speed of the
automobile can be
displayed
at
eye level on the windshield,
without distorting the field
of view. The driver no
longer
needs
to shift his eyes to the
instrument panel.
Electrochromic
devices
Active
electronic thin films
include high temperature superconductors,
conductive indium tin
oxide
(ITO) and vanadium
pentoxide, and ferro
electric barium and lead
titanates,
electrochromic
tungsten oxide, and titania
films used as phtotoanodes.
Solid state
electrochromic
(EC) devices for smart
windows, large area displays
and automotive
rearview
mirrors
are of considerable technological
and commercial
interest.
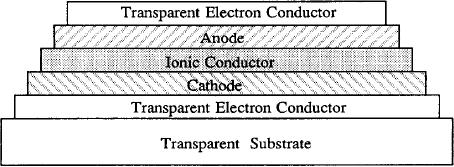
Fig.
5.12. Schematics of an EC window device
deposited on one substrate.
The
electrochromic
layer is anode, cathode or both
(reproduced from ref
.7).
Controlled
modulation of solar radiation
through smart windows has a
large potential in
buildings
for energy savings and in
automobiles to increase occupant comfort
while reducing
air
conditioning requirements. Typically
these devices consist of multiple
layer stacks that
are
sequentially
vacuum deposited or are
laminate constructions. Sol-gel
processing offers
advantage
in depositing films containing
multiple cations and
controlling the
microstructure.
These
parameters can influence the
kinetics, durability, coloring
efficiency and charge
storage
in the electrochromic films
(electrodes). A typical structure of a
solid state
electrochromic
window is shown in Fig.
5.12.
Other
applications of sol-gel techniques
include
Synthesis
of carbides sulphides and
nitrides
Synthetic
Strategies in Chemistry
5.17
Synthesis
of nanocomposites
Synthesis
of powders, grains and
spheres
Catalysis
( Mesoporous materials)
Preparing
solid electrolytes
Absorbing
coatings
Filters
for lighting and optical
purposes
Semiconducting
coatings
Protective
layers (both chemical and
thermal)
Scratch
resistant coating
Embedding
organic molecules (hybrid
organic-inorganic materials)
Flammable
gas sensors
Porous
gels and membranes
REFERENCES
1.
C. J. Brinker, and G. W. Scherer,
Sol-Gel Science: The Physics and
Chemistry of Sol-Gel
Processing,
Academic Press Inc., New
York, 1989.
2.
J. D. Wright and N.A.J.M
Sommerdijk, Sol-gel materials
chemistry and applications,
vol.4,
Taylor
& Francis Books Ltd.,
London, 2001.
3.
R. D. Gonzalez, T. Lopez and R.
Gomez, Sol-Gel preparation of
supported metal catalysts.
Catalysis
Today, 35 (1997) 293.
4.
L. C. Klein, (Ed.), Sol-Gel
Optics: Processing and Applications,
Kluwer Academic
Publishers,
Boston, 1993.
5.
L.L. Hench and J.K. West,
The sol-gel process. Chem.
Rev., 90 (1990) 33.
6.
B. L. Cushing, V.L. Kolesnichenko and
C.J. O'Connor, Recent Advances in
the Liquid-
Phase
Syntheses of Inorganic Nanoparticles.
Chem. Rev., 104 (2004)
3893.
7.
A. Agrawal, J.P. Cronin and
R. Zhang, Review of solid
state electrochromic
coatings
produced
using sol-gel techniques.
Solar Energy Materials and
Solar Cells, 31 (1993)
9.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES