 |
SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE |
| << METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method |
| SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE >> |
Chapter
- 4
SYNTHESIS
OF MATERIALS BASED ON SOLUBILITY
PRINCIPLE
C.
M. Janet
1.
INTRODUCTION
Fundamental
to the success of materials
science and technology is the
availability of
high-quality
materials exhibiting specific
tailor-made properties together
with an
appropriate
shape and microstructure. Solution
based methods especially
water based
mathods
offer numerous advantages. Cheap and
easy to handle precursors,
low cost,
simple
equipments, low energy input
and the eco-friendly nature
are few of them.
Moreover
they allow the easy
tailoring of synthesis parameters
throughout the whole
process,
which may be exploited to
achieve a more precise
control of composition,
shape
and
size of the resulting material. Since
the synthesis route
determines the properties of
the
material, the preparation
method chosen is very important
when designing
materials
for
specific applications. Wet
chemical routes for the
synthesis of nanostructures are
a
valuable
alternative to conventional processing and
gas phase synthesis, with
known
commercial
applications. Solvothermal methods and
hydrothermal methods
are
extensively
used in the synthesis of
materials of novel shape and
properties. The
majority
of
the metal organic frameworks
reported to date have been
synthesized using
solution
based
methods under bench-top
conditions (20-80 �C, 1
atm). Hydrothermal
synthesis
especially
has the attraction that it
favors the condensation of M-OH
into M-O-M bonds,
allowing
the preparation of materials
with multidimensional metal-oxygen
frameworks
[1].
Recognizing that most metal
salts are preferentially soluble in
polar solvents, for
example,
water, and that the opposite is
true for many organic
reactants, utilizing a higher
temperature
and biphasic solvothermal method
appears advantageous for
synthesizing
many
classes of hybrid materials.
Reaction at the interface of
two immiscible solvents
is
a
common technique for
crystallizing compounds at low to
moderate temperatures (<100
�C)
and is commonly used to prepare
hybrid inorganic-organic materials, in
addition to
other
products. The use of
biphasic solvothermal synthesis
has a number of
attractions
over
conventional hydro-/solvothermal methods.
The basic concept that
underlies in wet
4.2
Synthesis
of Materials Based on Solubility
Principle
chemical
methods or solvothermal methods
are the principles of
solubility. Hence, it is
important
to have a clear understanding of
the fundamentals of solubility to
that its use in
synthetic
procedures.
2.
BASICS OF SOLUBILITY
A
solution is a homogeneous mixture of two
or more substances. One of
the substances is
called
a solvent (a substance in which
other substance or substances
are dissolved). The
substances
dissolved in a solvent are
called solutes. A solution can
exist in a solid,
liquid
or
gas form depending on mixed
substances and external conditions
such as temperature
and
pressure. According to a chemists'
perspective solubility can be understood
as a
maximum
amount of solute that can
dissolve in a solvent at so called
equilibrium. In
chemistry,
equilibrium is a state where reactants
and products reach a balance which
means
that no more solute can be
dissolved in the solvent in
the set conditions
(temperature,
pressure). Such a solution is called a
saturated solution. There are
two
groups
of substances in case of which
solubility measure cannot be
applied. These are
miscible
and immiscible substances. Some
solvents, like water and
alcohol, can be mixed
together
and create a homogenous phase in
any proportion. A solubility
measure cannot
be
applied to such two
substances. Such substances
are called miscible. On the
other hand
if
two substances cannot be
mixed together (like water
and oil), they are
called
immiscible
[2].
In
the process of dissolving,
molecules of the solute are
inserted into a solvent
and
surrounded
by its molecules. For this
process to take place, molecular bonds
between
molecules
of solute as well as that of
solvent have to be disrupted.
Both of these require
energy.
For example when sugar
dissolves in water, new bonds
between sugar and water
are
created. During this process
energy is given off. The
amount of this energy
is
sufficient
to break bonds between molecules of sugar and
between molecules of
water.
This
example is relevant to any
solute and solvent. If the bonds
between the solvent
and
solute
are too strong and there is
not enough energy provided
to break them while
dissolving,
the solute will not
dissolve. The same energy
rule can be applied to salts.
Salts
composed
of positive and negative ions
which are bound together by
the force of
attraction
of their opposite charges. In cases where
energy needed to break their
ionic
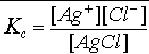
Synthetic
Strategies in Chemistry
4.3
bonds
is lower, the dissolution of salts can
take place only if the sufficient
energy is given
off
by an interaction of the ions
with solvent.
Salts
that are considered to be soluble
are Group I and ammonium
(NH4+)
compounds,
nitrates, acetates, chlorides,
bromides and iodides (except: silver
(Ag+),
lead
2+
(II)
(Pb2+),
mercury (I) (Hg2
),
copper (Cu+)
halides) and sulphates (except:
Silver
(Ag+), lead
(Pb2+), barium (II) (Ba2+),
strontium (II) (Sr2+) and
calcium(II) (Ca2+) ).
Those
which
are insoluble are carbonates
except Group I, ammonium
(NH4+) and
uranyl
compounds,
sulfites except Group I and
NH4+ compounds, phosphates
except Group I
and
NH4+ compounds, hydroxides and
oxides except Group I, NH4+,
barium (Ba2+),
strontium
(Sr2+)
and thallium (Tl+) and
sulfides except Group I,
Group II and NH4+
compounds.
The solubility principle
that holds well in the
case of organic compounds
is
"Like
dissolves like".
3.
SOLUBILITY PRODUCT (K SP)
Solubility
product constant is a simplified
equilibrium constant (Ksp)
defined for
equilibrium
between a solid and its respective
ions in a solution. Its
value indicates the
degree
to which a compound dissociates in
water. The higher the
solubility product
constant,
the more soluble the
compound is. The expression
for Ksp for a salt is
the
product
of the concentrations of the
ions, with each
concentration raised to a power
equal
to
the coefficient of that ion
in the balanced equation for
the solubility
equilibrium.
Solubility
product constants are used
to describe saturated solutions of ionic
compounds
of
relatively low solubility. A saturated
solution is in a state of dynamic
equilibrium
between
the dissolved, dissociated, ionic
compound and the undissolved
solid. For Silver
chloride
Kc is
given in the following
equation.
------------------
(1)
Where
[Ag+]
and [Cl-]
reperesent concentrations of ions of
Ag+ and Cl-
and [AgCl] is
a
value
representing the amount of
moles in a liter of solid
AgCl. [AgCl] is a constant
and
therefore,
the equation can be written
as
Kc
[AgCl] = [Ag+]
[Cl-]
---------------------
(2)
4.4
Synthesis
of Materials Based on Solubility
Principle
The
product of equilibrium concentartions of
Ag+ and
Cl- is equal to a constant.
This
constant
is called as solubility product
constant or Ksp [3].
3.1
Significance of Le Chatelier's Principle
in Solubility Concept
Once
a system has reached
equilibrium, the relative
concentrations or pressures of
the
species
in the reaction do not change.
However, if you disturb the
system in some way,
the
equilibrium will adjust until a
new equilibrium is established. Le
Chatelier's principle
states
that "If a system at equilibrium is
disturbed by a change in temperature,
pressure,
or
the concentration of one of the
components, the system will
shift its equilibrium
position
so as to counteract the effect of
the disturbance". The ionic
product (IP) is
simply
a measure of the ions present in
the solvent. This may
sound trivial but, in fact,
it
is
not always straightforward and
the concept opens up a
number of interesting
features
of
how salts behave in solution. The
product of the soluble ions
of a salt in solution is
called
the ionic product. The
solubility product (Ksp) is
the ionic product when
the
system
is in equilibrium. When the
ionic product exceeds the
solubility product,
precipitation
will happen according to the Le
Chatelier's principle. From
equation (2) this
can
be understood. Any kind of precipitation
is thus governed by the
Lechatlier principle.
One
of the day to day
significance of ionic and solubility
products are that they
are the
important
basic
chemical
phenomena
underpinning
the
tooth
mineralisation,
demineralisation
and stability. Addition of a common
ion affects the ionic
product. This
has
important implications in the
mouth since the concentration of
calcium and phosphate
ions
in saliva and plaque fluid can be
influenced by external factors.
Solubility principles
in
the case of gases are
quite different from that of
solids and liquids. As the
materials of
interest
are mostly in solid state
the solubility principles of
gases are not dealt
here.
3.2
Temperature and Pressure
Effects on Solubility
Heat
+ Solid sugar + Water = Dissolved sugar
------------ (3)
The
equation (3) represents two
processes: dissolution going
left to right, and
crystallization
going right to left. When
the sugar crystals are
dissolving at exactly
the
same
rate that sugar is crystallizing out of
solution, the system is at
equilibrium. The
balance
between dissolution and crystallization
can be changed by changing the
Synthetic
Strategies in Chemistry
4.5
temperature
of the solution. Adding heat will
favor dissolution. Cooling
the solution will
favor
crystallization.
The
temperature dependence of solubility is
also usually explained using Le
Chatelier's
principle.
Le Chatelier's principle predicts
that heating the solution
mixture will shift
the
equilibrium
in favor of dissolution, to remove
the added heat. This
explains why sugar is
more
soluble in hot water than in
cold. The solubility of a
substance is its
concentration
in
a saturated solution. Substances with
solubilities much less than
1 g/100 mL of solvent
are
usually considered insoluble. The
solubility is sometimes called
"equilibrium
solubility"
because the rates at which
solute dissolves and is deposited
out of solution are
equal
at this concentration.
The
solubility of solutes is dependent on
temperature. When a solid
dissolves in a
liquid,
a change in the physical state of
the solid analogous to
melting takes place. Heat
is
required
to break the bonds holding the
molecules in the solid
together. At the same
time,
heat
is given off during the
formation of new solute-solvent
bonds.
(a)
Decrease in Solubility with
Temperature:
If
the heat given off in the
dissolving process is greater than
the heat required to break
apart
the solid, the net
dissolving reaction is exothermic
(energy given off). The
addition
of
more heat (increases temperature)
inhibits the dissolving
reaction since excess heat is
already
being produced by the
reaction. This situation is
not very common where
an
increase
in temperature produces a decrease in
solubility.
(b)
Increase in solubility with
Temperature
If
the heat given off in the
dissolving reaction is less
than the heat required to break
apart
the
solid, the net dissolving
reaction is endothermic (energy
required). The addition
of
more
heat facilitates the dissolving
reaction by providing energy to break
bonds in the
solid.
This is the most common
situation where an increase in
temperature produces an
increase
in solubility for
solids.
The
use of first-aid instant
cold packs is an application of
this solubility principle.
A
salt
such as ammonium nitrate is
dissolved in water after a sharp
blow breaks the
containers
for each. The dissolving
reaction is endothermic - requires
heat. Therefore the
heat
is drawn from the
surroundings, the pack feels
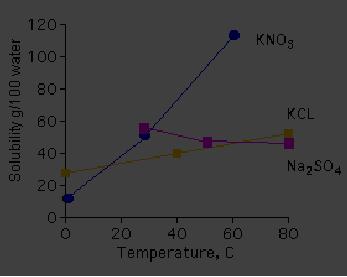
cold. The Fig. 4.1
represents the
4.6
Synthesis
of Materials Based on Solubility
Principle
effect
of temperature on the solubility of
three different salts.
Solubility of nitrate salt
of
K
is more compared to that of chlorides as
the temperature
increases.
Fig.
4.1. Temperature dependence of
solubility of different salts (ref.
2)
4.
HETEROGENEOUS EQUILIBRIA AND PRECIPITATION
Phase
transitions such as sublimation,
deposition, melting, solidification,
vaporization,
and
condensation are heterogeneous
equilibria, so are the
formation of crystals from
a
saturated
solution, because a solid and
its solution are separated
phases. The
equilibrium
constants
for saturated solution and solid
formation (precipitate) are
already defined as
solubility
product, Ksp. For
unsaturated and supersaturated solutions,
the system is not at
equilibrium,
and ion products, Qsp, which have
the same expression as
Ksp
is
used.
An
oversaturated solution becomes a
saturated solution by forming a solid to
reduce
the
dissolved material. The
crystals formed are called a
precipitate. Often, however,
a
precipitate
is formed when two clear
solutions are mixed. For
example, when a
silver
nitrate
solution and sodium chloride
solution are mixed, silver
chloride crystals
AgCl(s)
(a
precipitate) are formed.
Na+ and
NO3- are by-stander
ions.
Ag+(aq) + Cl-(aq) → AgCl(s)
(precipitate)
Silver
chloride is one of the few
chloride that has a limited
solubility. A precipitate is also
formed
when sodium carbonate is added to a
sample of hard water,
Ca2+(aq) + CO32-(aq) → CaCO3(s)
(precipitate).
Synthetic
Strategies in Chemistry
4.7
4.1
Solubility Products, Ksp,
and Ion Products Qsp
Formations
of precipitates are chemical
equilibria phenomena, and we usually
write these
heterogeneous
equilibrium in the following
manner, and call the
equilibrium constants
solubility
products, Ksp. If the
solution is not saturated, no precipitate
will form. In this
case,
the product is called the
ion product, Qsp.
4.2
Qsp, Ksp and
Saturation
For
some substances, formation of a
solid or crystallization does
not occur
automatically
whenever
a solution is saturated. These substances
have a tendency to form
oversaturated
solutions.
For example, syrup and honey
are oversaturated sugar solutions,
containing
other
substances such as citric
acids. For oversaturated
solutions, Qsp
is greater than
Ksp.
When
a seed crystal is provided or
formed, a precipitate will form
immediately due to
equilibrium
of requiring Qsp
to approach Ksp.
Sodium
acetate trihydrate,
NaCH3COO.3H2O,
when heated to 370 K will become
a
liquid.
The sodium acetate is said
to be dissolved in its own
water of crystallization.
The
substance
stays as a liquid when cooled to
room temperature or even
below 273 K. As
soon
as a seed crystal is present,
crystallization occur rapidly. In
such a process, heat is
released,
and the liquid feels warm.
Thus, the relationship among
Qsp, Ksp
and
saturation
is
given below [3]:
Qsp
< Ksp Unsaturated
solution
Qsp
= Ksp Saturated solution
Qsp
> Ksp Oversaturated
solution
5.
NANOPARTICLES THROUGH HOMOGENEOUS
NUCLEATION
For
the formation of nanoparticles by
homogeneous nucleation, a supersaturation
of
growth
species must be created. A reduction in
temperature of an equilibrium
mixture,
such
as saturated solution would lead to
supersaturation. Formation of metal
quantum
dots
in glass matrix by annealing at moderate
temperatures is a good example of
this
approach.
Another method is to generate a
supersaturation through in
situ chemical
reactions
by converting highly soluble
chemicals into less soluble
chemicals. For
example
semiconductor nanoparticles are
commonly produced by pyrolysis
of
organometallic
precursors. Nanoparticles can be
synthesized through homogeneous
4.8
Synthesis
of Materials Based on Solubility
Principle
nucleation
in three mediums: liquid,
gas and solid: however, the
fundamentals of
nucleation
and subsequent growth processes are
essentially the same. Before
discussing
the
detailed approaches for the
synthesis of uniformly sized monodispersed
nanoparticles,
it
is essential to review the fundamentals
of homogeneous nucleation and subsequent
growth
[4].
5.1
Fundamentals of Homogeneous
Nucleation
When
concentration of a solute in a solvent
exceeds its equilibrium
solubility or when
temperature
decreases below the phase
transformation point, a new
phase appears. A
solution
with solute exceeding the
solubility or supersaturation possesses a
high Gibbs
free
energy. The overall energy
of the system will be reduced by segregating
solute from
the
solution.
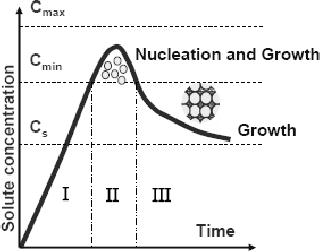
Fig.
4. 2. Nucleation and subsequent growth
(ref. 4)
When
the concentration of a solute
increases as a function of time no
nucleation would
occur
even above the equilibrium
solubility. Nucleation occurs
only when the
supersaturation
reaches a certain value above
the solubility. Homogeneous
nucleation is
important
for the formation of
particles of uniform size and
shape.
5.2
Examples of Nucleation
Pure
water freezes at -42 � C rather than at
its freezing temperature of 0 � C if no
crystal
nuclei,
such as dust particles, are
present to form an ice nucleus. Presence
of cloud
condensation
nuclei is important in meteorology
because they are often in
short supply in
the
upper atmosphere.
Synthetic
Strategies in Chemistry
4.9
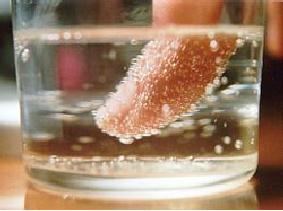
Fig.
4.3. Nucleation of carbon
dioxide bubbles around a
finger (ref. 5)
Nucleation
in boiling can occur in the
bulk liquid if the pressure
is reduced so that the
liquid
becomes superheated with respect to
the pressure-dependent boiling point.
More
often
nucleation occurs on the
heating surface, at nucleation sites.
Typically, nucleation
sites
are tiny crevices where
free gas-liquid surface is
maintained or spots on the
heating
surface
with lower wetting
properties. Substantial superheating of a
liquid can be
achieved
after the liquid is
de-gassed and if the heating surfaces
are clean, smooth and
made
of materials well wetted by
the liquid. The creation of
a nucleus implies the
formation
of an interface at the boundaries of the
new phase. Some energy is
consumed
to
form this interface, based
on the surface energy of
each phase. If a
hypothetical
nucleus
is too small, the energy
that would be released by
forming its volume is
not
enough
to create its surface, and
nucleation does not proceed.
The critical nucleus
size
can
be denoted by its radius, and it is when
r=r* (or r critical) that
the nucleation
proceeds
[5].
6.
SOLUBILITY AND CRYSTAL GROWTH
Crystal
formation is made up of three
phases: nucleation, growth, and
cessation of
growth.
During nucleation, the
slowest and most difficult
phase in crystal growth,
the
smallest
crystal capable of growth
forms. The barrier to
formation of these
smallest
crystals
results from differences in
the stability between
molecules at the core and those
on
the surface of the crystal.
During nucleation, the
average stability of a molecule in
a
crystal
is very low because the
attraction between the
molecule and the crystal is
often
less
than that between the
molecule and the solvent.
Thus, it is significantly easier
to
4.10
Synthesis
of Materials Based on Solubility
Principle
successfully
stimulate the birth of a
crystal by introducing a smaller
crystal (seeding)
than
by
merely supersaturating a solution.
Once nucleation has been
accomplished, the
growth
phase
of crystal formation begins, a phase
characterized by the addition of
molecules to
the
existing crystal. Two opposing
forces impact this process:
enthalpy and entropy.
Growth
of a crystal reduces the
entropy (a measure of randomness) of
the system because
a
molecule free in solution
has greater entropy than one
tethered to a crystal's
growing
surface.
Although entropy favors
dissolution of crystals, it is
energetically favorable for
a
molecule
to be added to a crystal. Above a
critical saturation point,
enthalpy overcomes
entropy,
and crystal formation can
occur.
6.1
Crystal Kinks and
Ledges
In
addition to temperature and
concentration, the geometry of a
growing crystal's
surface
influences
growth. Imagine a spherical
molecule approaching a planar
surface of a
crystal.
In this case, the addition
of the molecule is not
energetically favorable
because
there
is only one point of contact.
Next, consider a spherical
molecule approaching a
"ledge"
surface of a crystal. In this
case, the addition of the
molecule is more
energetically
favorable because the
molecule is stabilized by two
points of contact at
which
intermolecular forces hold
the molecule to the crystal.
In the third case, the
"kink"
structure
provides three points of
contact. The kink structure
is the ideal surface
geometry
for
crystal growth. Because
kinks are sparse on a
crystal's surface, the
addition of even a
small
amount of impurities that
effectively clog the kink
sites is capable of killing
crystal
growth.
In order for crystal growth
to be maintained, "ledges" or kinks
must remain
available
for molecules to be added to
the crystal. At one point in
the field of crystal
research,
scientists observed that nucleation
occurs too quickly to be
explained solely by
the
addition of molecules to a planar
crystal surface. As a result,
the spiral
dislocation
(SD)
theory arose, stating that
molecules are added to the
surface of a crystal in a
manner
resulting
in the creation of new
ledges or kinks so that
crystal growth can continue.
With
the
development of atomic force
microscopy (AFM) for
physically detecting changes
in
depth
on the atomic scale, it was possible to
study changes associated
with a ledge on a
crystal.
To observe crystal formation, the
lab uses an atomic force
microscope to gather
data
on the movement of ledges as
organic crystals grow and
dissolve. This method
allows
the investigator to observe the rate and
geometry of ledge movement at
various
Synthetic
Strategies in Chemistry
4.11
concentrations
of solute and in the presence of
impurities. It was noticed that
dissolution
is
not exactly the opposite of
growth. During dissolution,
the ledges are smooth
and
regular,
while they are rough and
irregular during growth.
Although there are
several
theories
on how the irregularity may
be conducive to crystal growth,
the differences have
not
been fully explained.
6.2
Practical Significance of Solubility
and Crystal Growth
Understanding
the growth and dissolution of
crystals is valuable in a variety of
contexts.
Crystallization
is an effective and economical
purification process, and, in
some cases
where
substances cannot be purified by
distillation, it is the only
practical method.
More
importantly,
approximately eighty percent of
all pharmaceutical agents
are pure
crystalline
solids. Understanding the
dissolution of a particular crystalline
drug could
allow
chemists to alter the drug's
dissolution rate. Moreover, understanding
the
conditions
of crystallization may allow
chemists to predict whether
drugs crystallize
within
the body. In 2004, the
journal Heart reported a
case of a large crystal
forming in
the
heart of woman given a
continuous dose of a drug
for ventricular tachycardia.
A
better
understanding of this drug's
crystallization could have
prevented the
incident.
Clearly,
much of this research is not
yet crystal-clear.
7.
SOLUTION BASED SYNTHETIC
STRATEGIES
Solution
based synthetic strategies
involve mainly sol gel
process, hydrothermal
synthesis,
solvothermal synthesis and reduction in
solution. Among which
solvothermal
synthesis
and hydrothermal synthesis are
discussed in detail in the present
chapter.
7.1
Solvothermal Synthesis
Solvothermal
synthesis utilizes a solvent
under pressures and temperatures above
its
critical
point to increase the
solubility of solid and to speed up
reaction between
solids.
Most
materials can be made soluble in
proper solvent by heating and
pressuring the
system
close to its critical point.
This method allows the
easy control on the
solubility of
a
solute. And it leads to
lower super saturation state
which is necessary for
the
precipitation
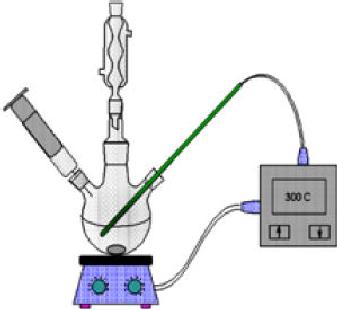
to happen. The reaction set-up
used for solvothermal
synthesis is given in
Fig.
4.4.
4.12
Synthesis
of Materials Based on Solubility
Principle
Fig.
4.4. Solvthermal synthesis
set-up (ref. 6)
7.1.1
Synthesis of Semiconductor
Chalcogenides
In
general, semiconductor chalcogenides of
different sizes and shapes
are prepared using
solvothermal
synthesis. In our laboratory we
have synthesized CdS
nanostructures
especially
nanorods through solvothermal
synthesis using a solid
precursor CdC2O4.
The
reagent
used to precipitate CdS was
(NH4)2S. The
reaction observed was represented
in
the
equation 6. Here the solvent
used was ethylene glycol and
the reaction
temperature
was
60 �C
and the reaction was carried
under a constant flow of an
inert gas [7].
CdC2O4
+ (NH4)2S
CdS
+ (NH4)2C2O4
----------
(6)
CdC2O4
is an
insoluble solid and (NH4)2S was liquid.
But the solvothermal
synthesis at
low
temperatures allowed in this case a
slow replacement reaction of
the ligand forming
the
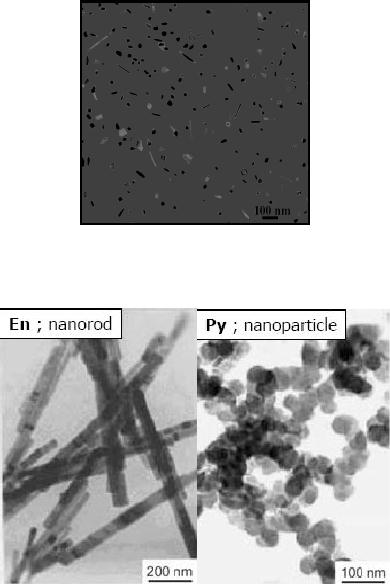
nanostructures of CdS. The TEM images of
the CdS nanorods are
given in the Fig.
4.5.
By changing the solvents,
temperature and the precursor
variety of sizes and
shapes
can
be produced through this
method. When ethylene
diamine is used the material
formed
was
also found to be nanorods with
high aspect ratio. But,
when pyridine was used
CdS
nanoparticles
are only formed.
Synthetic
Strategies in Chemistry
4.13
Fig.
4.5. TEM image of CdS prepared
from CdC2O4 using
solvothermal method in
ethylene
glycol (ref. 7)
Fig.
4.6. TEM image of CdS prepared
from CdC2O4 using solvothermal method
in
ethylene
diamine and pyridine (ref.
6)
7.1.2
Synthesis of Metal
Nanoparticles
Pt
and Pd nanoparticles were synthesized by
microwave-assisted solvothermal
method.
The
only difference is the
heating source is microwave. PVP with an
average molecular
weight
of 40'000 was used as a capping agent in
the experiments. H2PtCl6,
and
Palladium(II)2,4-pentanedionate
were used as metal
precursors. PVP was dissolved in
methanol
or ethanol and then the
metal salts were added. The
reactants were heated
for
60
min at 90 �C when
methanol was used as a reducing agent and
at 120 �C
when
ethanol
was used as a reducing agent for 60
min under microwave
irradiation [8].
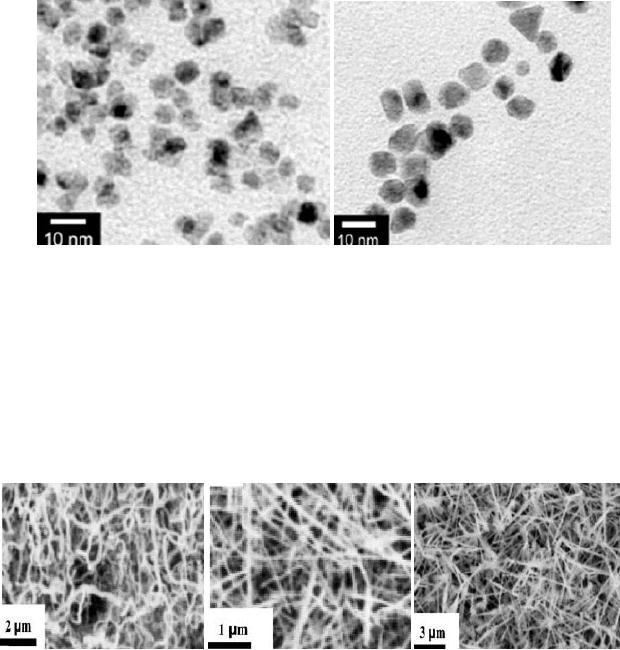
4.14
Synthesis
of Materials Based on Solubility
Principle
Fig.
4.7. TEM images of the
obtained Pt and Pd nanoparticles
under
microwave-assisted
solvothermal conditions (ref.
8).
7.1.3
Synthesis of Metal Nitrides
and Oxides
In
another approach single source precursors of
halides of Al, Ga and In were
prepared
with
urea complexation and further
solvothermal synthesis at higher
temperatures
resulted
in the formation of the
respective nitrides. These nitrides
are of special relevance
in
photovoltaic applications
[9].
(a)
(b)
(c)
Fig.
4.8. TEM images of (a) AlN
(b) GaN (c) AlN nanowires
formed during
solvothermal
synthesis
(ref. 9).
A
new and facile route was developed by
Suib et al., to manipulate
the growth of
hierarchically
ordered Mn2O3 architectures via a
solvothermal approach. Various
solvents
are
employed to control the
product morphologies and structures.
Mn2O3
with
unique
cuboctahedral,
truncated-octahedral, and octahedral
shapes are obtained. In a
typical
synthesis
Mn(NO3)2 was dissolved in an organic
solvent followed by a vigorous
stirring
at
room temperature for half an
hour in a Teflon liner. Then
the Teflon liner was
transferred
and sealed in an autoclave for
solvothermal treatment at 120 �C for 20
h. A
Synthetic
Strategies in Chemistry
4.15
variety
of different solvents was used to
investigate the effect of
solvents on the
morphology
of the resultant Mn2O3.
In order to investigate the
development of Mn2O3
crystals,
the reactions were also
conducted at different temperatures using
ethanol as the
solvent.
FESEM images of the Mn2O3
synthesized in
different solvents and for
different
duration
are given in Fig. 4.9 and
Fig. 4.10 respectively. The
images indicate the
shape
evolution
of Mn2O3 polyhedra. Mn2O3
is used in
catalytic as well as
electrocatalytic
applications
[10].
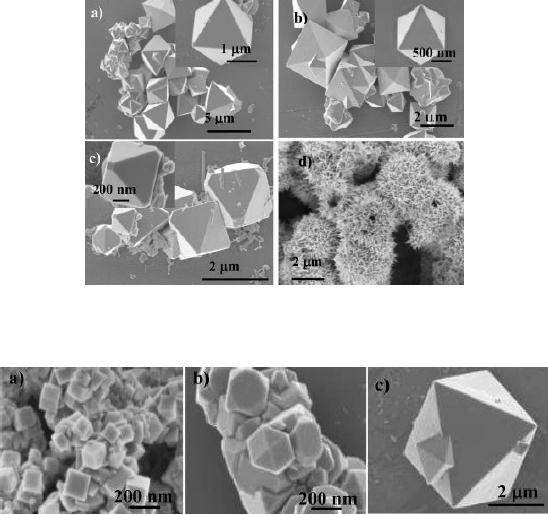
Fig.
4.9. FESEM images of
products synthesized in
different
solvents:
(a) ethanol, (b) 1-butanol,
(c) 2-butanol, and (d)
acetone (ref. 10).
Fig.
4.10. FESEM images of
products synthesized under
different
reaction
periods (a) 1.5 h, (b) 2 h, and
(c) 3 h (ref. 10).
7.2
Hydrothermal Synthesis
Hydrothermal
synthesis as the name indicates
the solvent is always water.
If liquid water
is
placed in an open container, its
temperature cannot be raised above 100
�C. But, if
water
is heated in a sealed container, it can
be heated to temperatures above 100 �C
which
means that supercritical
properties of the water can be
utilized under this
4.16
Synthesis
of Materials Based on Solubility
Principle
condition.
The advantages of inducing
supercritical behavior in hydrothermal
synthesis
are
that it will provide a single
phase behavior and give enhanced
permeability, mass
transport
capability and dissolving capacities.
Hydrothermal synthesis can be defined as
a
method
of synthesis of single crystals
which depends on the
solubility of minerals in
hot
water
under high pressure. Hydrothermal
synthesis is a century old
synthetic strategy and
it
was used for the synthesis
of minerals in general. From
80's hydrothermal method
was
utilized
extensively for newer
material synthesis. Advantages of
hydrothermal synthesis
are
in this method no post-heat
treatment is needed and hence
agglomeration will be less.
After
preparation no milling is required
which will reduce impurities. Any
complex
chemical
compositions can be synthesized by using
this method. Particle size or
shapes
can
be controlled in this approach. It can
induce self assembly leading
to newer and
complex
architectures of materials and the
precursors used are relatively
cheap raw
materials.
And hydrothermal method
crystal growth includes the
ability to create
crystalline
phases which are not stable
at the melting point. Also,
materials which have
a
high
vapour pressure near their
melting points can be grown by
the hydrothermal
method.
The
method is also particularly suitable
for the growth of large
good-quality crystals
while
maintaining good control over
their composition. Disadvantages of the
method
include
the need of expensive
autoclaves, good quality seeds of a
fair size and the
impossibility
of observing the crystal as it
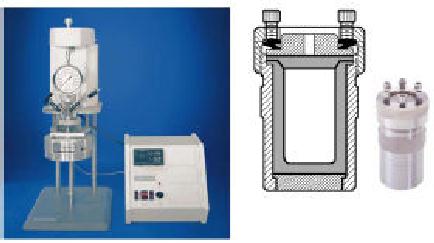
grows. Fig. 4.11 shows
the reaction set-up
for
hydrothermal
synthesis. The pressure
generated inside the reactor
can be read from the
pressure
gauge.
Fig.
4.11. Hydrothermal reactor
set-up (ref. 6)

Synthetic
Strategies in Chemistry
4.17
7.2.1
Synthesis of Oxides and
Mixed Oxides
Barium
titanate (BaTiO3)
perovskite is widely used in
electronic industry in
multilayered
ceramic
capacitors due to its high dielectric
constant. Hydrothermal synthesis
route is
promising
due to homogeneity, exact stoichiometry
and spherical morphology of BT
powders
obtained by this synthesis
method at low temperature
(<200 �C). Using
commercially
available titania as Ti precursor
(Degussa P-25) and Barium
hydroxide
precursor
in the Ba: Ti = 1:1 ratio at
a temperature as low as 120 �C for 48
h yielded
BaTiO3 crystals [11]. The
SEM image of BaTiO3 prepared is given in Fig.
4.12.
Fig.
4.12. SEM image of
BaTiO3 crystals (ref.
11).
Alkali
treatment of commercially available
TiO2 (Degussa p-25) in
hydrothermal
conditions
(130 �C) will
result in the formation of
nanotubes of TiO2.
These tubes
obtained
on further hydrothermal treatment at a
high temperature of around 175
�C
will
yield
nanorods of TiO2 [12]. Thus hydrothermal
synthesis provides a route to
play around
different
morphologies of the same
material by simply changing
the temperature.
Hydrothermal
treatment of zinc chloride
hydrazene hydrate at 140 �C for 12
h
resulted
in the formation of flower
like microrod bundles. The
solution phase is
accelerating
the process of self assembly
through a
dissolution-recrystallization-
decompositiongrowth
process [13]. The chemical
reactions are represented in
the
following
equations. ZnO has variety
of applications in photonics and
optics.
ZnCl2 +
2N2H4
ZnCl2(N2H4)2 ..................(1)
Zn2+ +
2NH3.
H2O
Zn(OH)2+
2NH4+
................(2)
Zn(OH)2
ZnO
+ H2O
......................(3)

4.18
Synthesis
of Materials Based on Solubility
Principle
Fig.
4.13. FE-SEM images of (a)
precursor ZnCl2(N2H4)2 obtained at room
temperature
(b)
ZnO samples obtained at 140 �C and 12 h
(ref. 13).
7.2.2
Synthesis of Noble Metal
Architectures through Hydrothermal
Synthesis
Polymer
protected (PDDA-poly (diallyl
dimethylammonium) chloride)
noble-metal
(including
silver, platinum, palladium, and
gold) nanostructures in the
absence of any
seeds
and surfactants can be synthesized using
hydrothermal method in which
PDDA, an
ordinary
and water-soluble polyelectrolyte, acts
as both a reducing and a
stabilizing
agent.
Under
optimal
experimental
conditions,
Ag
nanocubes,
Pt
and
Pd
nanopolyhedrons,
and Au nanoplates are obtained. In
typical synthesis, PDDA
along with
the
respective precursors such as
AgNO3 (170 �C for 16 h),
H2PtCl6
(140 �C
for 40 h),
H2PdCl4
(190 �C
for 40 h) and HAuCl4 (170 �C for 12 h) at
specific pH was used
[14].
Fig.
4.14. Typical SEM and TEM
images of the PDDA-protected
(a) Ag nanocubes (b) Pt
nanopolyhedrons
(c) Pd nanopolyhedrons and (d) Au
nanoplates (ref. 14).
Synthetic
Strategies in Chemistry
4.19
Apart
from metals and metal oxides
hydrothermal synthesis method is
used in the
preparation
of zeolites and mesoporous materials.
This method was also found to
be
effective
in the synthesis of different carbons
such as nanotubes, fullerene and
diamond
[15].
7.2.2
Synthesis of Polymeric Materials
through Hydrothermal
Synthesis
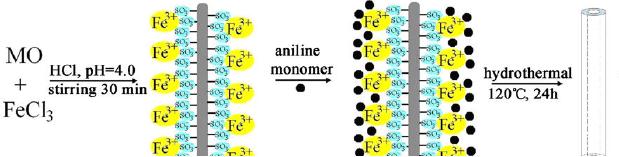
Polyaniline
(PANI) mesostructures have
been synthesized under
hydrothermal
conditions.
The mesostructures show
different forms - fibers,
dendrite fibers,
textured
plates,
featureless plates, and spheres [16]. In
a typical synthesis, a complex
of
FeCl3�6H2O and methyl orange in
hydrochloric acid aqueous solution
(pH = 4.0) was
stirred
and transferred to an autoclave with
aniline monomer and kept at 120 ◦C
for 24 h.
The
material collected was found to be PANI
nanotubes [17]. The
formation mechanism
and
the TEM images of the tubes
formed are given in Scheme 1
and Fig. 4.15. A
fibrillar
complex
of FeCl3 and methyl orange (MO)
acting as reactive selfdegraded templates
in
hydrothermal
conditions was the driving
force for the growth of
nanotubes. MO, which
contains
a hydrophilic group
(SO-3), possesses an anionic
characteristic when
dissolved
in
water. It could dimerize at a
particular concentration to form
higher oligomers.
When
aniline
monomer was added into the
solution, polymerization occurred on
the surface of
MO
where the oxidant
FeCl3 was adsorbed and MO itself
degraded automatically
during
the
polymerization process.
4.20
Synthesis
of Materials Based on Solubility
Principle
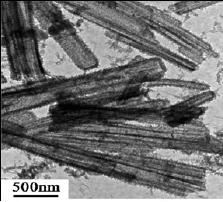
Fig.
4.15. TEM of products with pH of
electrolyte (4.0) Synthesis
conditions:
temperature,
120 ◦C;
FeCl3,
1.5 mmol; MO, 0.075 mmol;
aniline, 1.5 mmol (ref.
17).
CONCLUSIONS
The
solubility of a material is having a
major role while designing
the synthetic
strategy
of
any new materials. Solution
phase synthesis always
assisted the self assembly
and
gradual
growth of the crystals of a
variety of materials ranging
from metals, metal
oxides,
chalcogenides,
polymers, zeolites and carbon
materials. Easy tailoring of the
morphology
and
properties can be achieved if solubility concepts
are suitably exploited in
new
material
synthesis. Solution based
chemistry is always important
because ultimate
utility
of
materials is going to be in any
form of life chemistry which
is fully based on aqueous
systems.
Drug delivery materials,
materials in food processing and
preservation,
medicines
and cosmetics are much trivial
cases where the solubility
concepts are
extremely
important. Hence, immense
care should be taken while
designing materials
for
day
today applications through
solution chemistry.
REFERENCES
1.
P. M. Forster, P. M. Thomas and A. K.
Cheetham, Chem.
Mater.,
14 (2002)17.
2.
http://www.elmhurst.edu/~chm/vchembook/174temppres.html
3.
http://research.yale.edu/ysm/article.jsp?articleID=354
4.
Nanostructures and nanomaterials,
Synthesis, Properties and applications,
Cao (Editor)
(2004),
Imperial College press,
London.
5.
en.wikipedia.org/wiki/Nucleation
6.
D. K. Kim Nanoparticle lecture,
2003.
7.
C. M. Janet and R. P. Viswanath, Nanotechnology,
17
(2006) 5271.
Synthetic
Strategies in Chemistry
4.21
8.
Q. Lu, F. Gao, D. Li and S. Komarneni,
A to Z of
Journal of Materials online,
1(2005)
1.
9.
K. Sardar, M. Dan, B. Schwenzer and C. N. R.
Rao, J.
Mater. Chem.,
15 (2005) 2175.
10.
W.-N. Li, L. Zhang, S.
Sithambaram, J. Yuan, X.-F.
Shen, M. Aindow and S. L.
Suib,
J.
Phys. Chem. C.,
111 (2007)
14694-14697.
11.
http://www.isotec-cluster.at/internal/uploads
12.
J.-N. Nian and H. Teng, J. Phys.
Chem. B 110
(2006) 4193.
13.
C. Jiang, W. Zhang, G. Zou, W. Yu and Y.
Qian, J.
Phys. Chem. B. 109
(2005) 136.
14.
H. Chen, Y. Wang, S. Dong, Inorg.
Chem. 46
(2007) 10587.
15.
http://www.sawyerresearch.com/Newsletters/May03newsletter.pdf
16.
L. J. Pan, L. Pu, Y. Shi, T.
Sun, R. Zhang and Y. O. Zheng,
Adv.
Fun. Mater. 16
(2005)
1279.
17. H.
Huang, X. Feng and J.-J. Zhu, Nanotechnology, 19
(2008) 145607.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES