 |
SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY |
| << NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC |
| SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice >> |
Chapter
- 13
SYNTHETIC
STRATEGIES - FROM LABORATORY TO
INDUSTRY
S.
Chandravathanam
INTRODUCTION
Scale-up
is an act of transferring a laboratory
process to the larger
equipment typical of a
commercial
plant, or designing a piece of
commercial equipment based on
research-scale
models.
This is often a complex
matter in which, for some
processes, trail and error
still
has
a significant foothold. Even
with careful planning and
strict methodology,
scale-up
can
be fraught with difficulty and
unexpected problems. The
reasons for this
are
numerous;
many common laboratory
methods cannot be applied at
the large scale,
equipment
may exhibit unexpected
behaviour at sizes never
used before, or critical
heat
or
mass transfer phenomena may
not be discernible at laboratory
scale.
The
design of a new plant or
commercialization of a new chemical
process represents
a
tremendous investment of time and
money. The risk is considerable and
the economic
penalty,
if the plant or process
fails to produce as expected is severe.
To minimize such
risks,
industries undertake lengthy and
expensive process research and
development
programs.
An
invention might sometimes lay
unused for ages without
paying back in terms of
industrial
realization and of profitable business
for its lack of technical
know-how. For
example,
the pigment indigo was
extracted from the sea
animals during the 13th century.
One
gram of the pigment could be
obtained from almost 10,000
numbers of the
animal.
So
that it was the very
expensive dye material, and
the colour was restricted to
the Royal
family
only, and so named as the Royal
purple. It was the situation
till Bayer in the 19th
century
studied the dye material,
and started synthesizing it from the
easily available raw
materials.
This
text deals with the
technical knowledge and on the
tools that are necessary
to
change
an invention into a true
innovation. The term
scale-up has usually been
explained
as
how to design an industrial reactor able
to replicate the results
obtained in the
laboratory.
These are done with most of
the time innovative ideas,
and sometimes with
13.2
Synthetic
Strategies From Laboratory to
Industry
many
mistakes.The scale-up usually
means that the scaling
facor of 1000 times from
the
laboratory
process to the pilot plant
scale, and further 1000 times
from the pilot to
the
industrial
scale. Typical laboratory
scale preparation lies with
in the production of 100
g/day.
A pilot plant will typically
produce 1-50 Kg/day, and the
industrial plant will be
aimed
with the production of
tons/day.
Few
of the indispensable steps which
are undertaken while going
from the laboratory
scale
to the commercial or industrial
scale are, cost estimation,
process design, pilot
plant
studies
may or may not be
accompanying with the
dimensional analysis.
COST
ESTIMATION
The
cost estimation for the
scale-up of production is a very
crucial step. For this
process,
chemical
engineers long been relying as a
rule a thumb on the use of
power law. As per
the
law the investment is
proportional to the scale of a
production facility raised to
some
constant
power, characteristic of the
particular process.
The
so called power law of
investment takes the
form,
I2/I1 =
k (Vt,2/Vt,1)1/n
Where
I is investment, Vt is
production rate and k and n are
constants.
The
power law is based on the
`minimization law', which
states that people
minimize
their
efforts per unit of dimension
whenever a change of scale or volume is
required. The
constant
n is the number of dimensions of
the production rate-limiting
activity, typically
is
either one, two or three
corresponding to the linear,
area and volumetric
dimensions.
PROCESS
DESIGN
Process
Design is the heart of the
process of scale-up. When
the research
department
discovers
a new reaction to make an existing
product or a new material,
the process
department
will have to translate these
discoveries into a new
process which could
be
commercially
and technically feasible.
The
few of the factors of
importance during this
process design stage
are,
-
Production rate
-
Temperature and pressure conditions of
the reaction
-
Every available information
about operating variables,
thermodynamics and kinetics of
the
chemical reaction
-
Stoichiometry of the main
reaction
Synthetic
Strategies in Chemistry
13.3
-
Desired yield of the
product
-
Desired product purity and the
possibility of the feed
recycling
The
importance of the process design
step can be explained with one of
the case studies
with
the process design for the
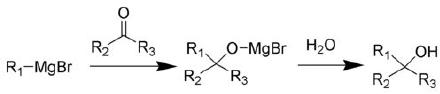
manufacture of Grignard Reagent. Grignard
reagent is one
of
the important reagents in
organic synthesis for the
introduction of alkyl groups
on
carbonyl
carbon atoms. It's
requirement is vast but its
manufacturing in large scale
was
not
undertaken for a long time
for some reasons like
the high exothermicity of
the
reaction,
the high inflammability of
the solvent diethyl ether,
and the high sensitivity
of
the
reagent for water, which
needs to be used for cooling
purpose.
Grignard
reagent is prepared in situ when
alkyl halide reacts with Mg
metal scraps in
diethyl
ether.
Through
careful process design studies, these
drawbacks had been overcome;
with the
use
of alkyl chlorides or bromides instead of
iodides, the exothermicity of the
reaction
can
be reduced as a result of the reduction
in the reaction rate of chlorides and
bromides
compared
to their iodide counterpart.
Along with that, it could
also reduce the cost as the
chloride
is cheaper than the iodide.
The risky diethyl ether was
replaced with
tetrahydrofuran
(THF), which has atleast 30 �C
higher boiling point than
the former. Inert
gases
which can play both as the
cooling system and blanket replaced
water, as the
product
Grignard reagent is more sensitive to
water, if there is any
leakage.
PILOT
PLANT
A
pilot plant is generally a
collection of equipment designed to
allow operation of a
novel
process
at a scale small enough to be
safely manageable but large
enough to provide a
realistic
demonstration of operations and physical
principles as they might
apply in a
commercial
facility and to allow the
collection of meaningful engineering
data for a
further
scale-up.
Pilot
plants provide important
information on the best ways
to handle reactants,
intermediates,
products and waste streams, on energy
transfer, on the best choice
of
13.4
Synthetic
Strategies From Laboratory to
Industry
separation
technologies and an operating procedures.
Some of the many areas of
process
development
in which a pilot plant can
play an important role
are,
-
Confirming the operational
feasibility of a new
process
-
Identifying scale-up effects on
yield and selectivity
-
Collecting kinetics and design
data
-
Testing materials of
construction
-
Testing the operability of
control schemes
-
Assessing process hazards and safety
issues
-
Commercial viability of the
raw material
-
Process troubleshooting and
optimization
-
Testing process recycle
streams
Pilot
plants can be classified according to
numerous criteria. Foremost is
the fundamental
distinction
between a pilot plant for a
continuous process versus one
for a batch or
semibatch
process. In the continuous
process, pilot plants tend
to be single-purpose,
product
dedicated facilities that
are generally smaller. Batch
pilot plants, typical of
the
fine
chemical industry, tend to be
multipurpose. The requisite
flexibility to handle a
wide
variety
of products and processes can add
considerably to the complexity and cost
of a
plant.
DIMENSIONAL
ANALYSIS
Dimensional
analysis is a process by which
the dimensions of equations and
physical
phenomena
are examined to give new
insight into their
solutions. It is a powerful
technique
for spotting errors in
equations. It shows how
physical dimensions of
the
variables
that govern a problem can be
used to find physical laws.
The main requirement
for
dimensional analysis is dimensional
homogeneity it states that
all parts of an
equation
must have the same
dimension. In other words, we can
add or compare
quantities
that have similar dimensions
only.
Dimensional
analysis results in many
advantages like
-
the
reduction of number of variables in a
variable set; if there are `n'
variables or
parameters
of concern, then the total
number of dimensionless numbers is
reduced
to
n - r, where `r' is the number of
basic dimensions of all the
parameters of
concern
(Buckingham pi theorem).
Synthetic
Strategies in Chemistry
13.5
-
Gives
the guidelines for scaling
the results from model test
to the full-scale.
Otherwise
dimensional analysis sets
the rule under which full
similarity in model
test
can be achieved.
-
Nondimensional
parameters are more
convenient than dimensional
parameters
since
they are independent of the
system of units.
In
the following passage two
case studies of application of
dimensional analysis
are
given.
CASE
STUDY 1: Correlation between Meat Size
and Roasting Time
What
is the roasting time for
two times the mass of
the meat?
The
main parameters of concern
for this problem are
listed below along with
their
dimensions.
Symbol
dimension
Physical
quantity
T
roasting
time
θ
L2
A
meat
surface
thermal
diffusivity
a
L2T-1
T0
surface
temperature
Θ
temperature
distribution
T
Θ
The
higher the heat conductivity k, of
the meat, the faster is
the cooking.
The
higher the heat capacity ρCp,
the slower is the heat
transfer.
∴Thermal
diffusivity a = k/ρCp
The
total number of dimensionless
numbers are 5-3 = 2.
∏1
= T/T0
(T0-T)/T0)
or
F0
∏2
= aθ/A
When
the temperature distribution
T/T0 is
achieved throughout the meat
then it can be
said
that the meat is cooked.
T/T0 =
f
(F0)

13.6
Synthetic
Strategies From Laboratory to
Industry
F0
aθ/A
= idem
T/T0 =
idem
θ
α A
idem = identical
This
equation relates the roasting
time with the area of
the meat. But in reality
the
roasting
time needs to be calculated
with respected to the mass
of the meat.
mass
is related to area with the
following equation.
m
= ρV
α
ρL3 α
ρA3/2
the
density ρ
remains
the same irrespective of the
meat size. Therefore,
ρ
= idem
and
A
∝
m2/3
θ2/θ1 α
(m2/m1)2/3
That
is,
θ
α m2/3 =
m0.67
∴doubling
the mass of meat, the
roasting time increases by,
22/3 =
1.58 times. The
final
equation
contains only two parameters, instead of
the initial five parameters,
therefore,
the
number of supplementary experimental
runs is getting drastically
reduced.
CASE
STUDY 2: Homogeneous Irreversible 1st Order Reaction in a
Tubular
Reactor
How
much is the volume and
residence time of the
reactor to be increased for
the
increase
of volume throughput by a factor of n
(qT =
nqM)?
The
important parameters of concern
are, v - flow rate, d, L diameter
and length of the
tubular
reactor, ρ,�
-
fluid density and viscosity
respectively. T0
inlet temp.
cin,
cout inlet and
outlet conc. keff.
effective
reaction rate constant
keff. =
k0 exp(E/RT)
The
parameters of mass and heat transfer
are, D - Diffusion coefficient, Cp
heat
capacity,
k - thermal conductivity, cin ĆHR - heat of
reaction per unit time and
volume,
T0 -
inlet temp., ĆT - temp.
difference between fluid and
tube wall.
The
complete relevant list of
parameters is,
v,
d, L, ρ,
�, cout,
cin, k0,
E/R, D,
Cp, k, cinĆΗR,
T0, ĆΤ
Synthetic
Strategies in Chemistry
13.7
From
the pi theorem, the number
of nondimensional parameters should
be, 15 - 6 = 9.
The
obvious five nondimensional
numbers are,
L/d,
Cout/Cin, E/RT0
and
ĆΤ/Τ0
The
remaining four nondimensional
numbers are derived using
the following
combination
of the parameters.
=
(k0 τ)-1 (mean residence time
τ
L/v
at pipe flow)
Π1
= v/Lk0
Π2
= �/ρd2k0
=
(k0 τ
Re
L/d)-1
Π3
= D/L2k0
=
(k0 τ
Re Sc
L/d )-1
=
Da-1 (Da
= Damkohler number)
Π4
= (ρCpΤ0) / cin ĆΗR
Π5
= (kΤ0) / cin ĆΗR
d2 k0
= (k0 τ
Re Pr Da
L/d)-1
∴The
nine dimensionless numbers
are,
L/d,
Cout/Cin,
E/RT0,
ĆΤ/Τ0, κ0τ,
Re,
Sc, Pr, Da
During
scale up
�
No change of reaction temp. ∴T0 and k0
are
constant
�
No change in the physical and chemical
partners of the reaction ∴the
kinetic and
material
numbers, remain
unchanged.
�
L/d =
identical
�
To attain specified degree of conversion,
cout/cin = identical
Therefore,
the following two are
the only two parameters
need to be adjusted.
(k0 L)/v
Re
= (v d ρ) /� and
k0τ
But
it is impossible to have
both,
vd
v
L = idem and L/v = idem and L/d =
idem
For
the given condition of
qT
= nqM, , dT
= ndM,
q
∝
vd2 and vTdT2 = n
vMdM2
13.8
Synthetic
Strategies From Laboratory to
Industry
as,
Re α
v d =
idem
VT =
(VM)/n
VT =
n3 VM
and
hence τΤ = n2 τΜ (τ = V/q)
Where,
M
- model scale
T
- technological or industrial
scale
q
volume throughput
n
factor
V
volume of the
reactor
The
results state that for
the increase of volume
throughput n times the
volume of the
reactor
needs to be increased by n3 times
and the residence time needs
to be increased by
n2 times.
REFERENCES
1.
Kirk-Othmer, Concise Encyclopedia of
Chemical Technology, Fifth
Edition, Vol. 2,
John-Wiley
& Sons, 2007.
2.
John J. Mcketta (Ed.,),
Encyclopedia of Chemical Processing and
Design, Vol. 49,
1994.
3.
Marko Zlokarnik, Scale-up in
Chemical Engineering, , Wiley-VCH,
2002.
4.
Keld Johansen, `Aspects of scale-up of
catalyst production', Studies in
Surface
Science
and Catalysis, 143 (2002) 1.
5.
Gianni Donati, Renato
Paludetto, Catalysis Today, 34
(1997) 483.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES