 |
ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS |
| << THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible |
| NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC >> |
Chapter
- 11
ELECTROCHEMICAL
SYNTHESIS
M
Helen
INTRODUCTION
Electrochemistry
is a
branch of science which
deals with electrical energy
and chemical
change.
Spontaneous chemical reactions liberate
electrons and they are taken
place in
Galvanic
or Voltaic Cells.
These cells are exploited in batteries
and fuel cells to
produce
electric
power. On the other hand
Electrolytic
Cells are
nonspontaneous where
electrical
energy
is required to carry out
chemical transformations. For an
example in the
process
called
water electrolysis electrical
energy is supplied to split
water in producing
hydrogen
and
oxygen. Electrolysis is exploited in
electroplating, chlorine gas
production and in
refining
metals.
Electrochemical
synthesis is
the production of chemical
products or
materials
using electricity as the
driving or controlling factor.
Electrochemical synthesis
is
achieved by passing an electric
current between two electrodes
separated by an
electrolyte.
That is, the synthesis
takes place at the electrode-electrolyte
interface. This
method
has significant potential
for improving availability of
specialty products and
for
improving
environmental compatibility in several
industrial sectors by destroying
or
converting
unwanted byproducts into
useful products. Much
progress has been made
in
the
last few decades in
advancing the basic
understanding and industrial applications
of
electrochemical
processes, but many aspects
of electrochemical synthesis are
still
inadequately
understood or explored.
FEATURES
OF ELECTROCHEMICAL SYNTHESIS
The
features that distinguish
electrosynthesis from other
synthetic methods are:
The
experiments are simple to
perform and the instruments
required are
inexpensive
and readily available.
Electrochemical
synthesis takes place at the
electrode-electrolyte interface
which
has
a very high potential
gradient of 105 V
cm-1.
Under
these conditions, the
reactions
often lead to products which
cannot be obtained in a
conventional
chemical
synthesis.
An
electrochemical synthesis is a redox
reaction. By fine-tuning the
applied cell
potential,
the oxidizing or reducing
power can be continuously varied.
This
11.2
Electrochemical
Synthesis
possibility
of continuous variation is not
available in conventional
chemical
synthesis.
The
product is normally deposited on
the electrode in the form of
a thin film or a
coating.
The
film composition can be controlled by
varying the bath
composition.
Electrochemical
synthesis is a low-temperature process
limited by the
boiling
point
of the electrolyte.
Reaction
taking place can be controlled
kinetically by controlling the
current
passed
through the cell, while it
can be thermodynamically controlled by
choosing
the
applied potential.
In
summary, electrochemical synthesis is a
`green' route to produce to
fabricate high-
purity
materials without any
additives.
ELECTROCHEMICAL
SYNTHESIS: DESIGNING
Any
electrochemical reaction depends on
the proper choice of a
number of reaction
parameters
such as:
(1)
Choice of an electrode
(2)
Choice of an electrolyte
(3)
Choice of temperature, pH,
concentration, and composition of the
electrolyte
solution
(4)
Choice of the cell design -
divided or undivided
(5)
Mode of electrolysis - potentiostatic or
galvanostatic (constant potential
or
constant
current)
In
a typical electrosynthesis, the
reactant, which is dissolved in
the electrolyte is
deposited
as a solid product.
When
a metallic salt is dissolved in
water it dissociates to
form
positively charged ions. The
solution that contains these
charged ions is referred to
as
an electrolyte or a plating solution. By
passing electric current
through this
electrolyte,
one
can reduce the metal ions to
form solid metal. This
process is referred to
electroplating
or
electrochemical
deposition.
In Fig. 11.1 electroplating is
explained by
taking
silver as the anode, fork as
the cathode (fork to be plated
with silver) and
aqueous
solution
of silver nitrate as an electrolyte.
Both anode and cathode are
connected to the
external
battery. When the external
power is on silver metal at
the anode is oxidized
to
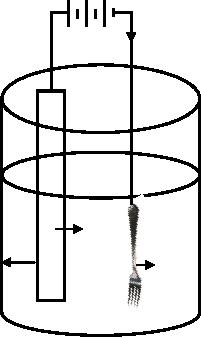
Synthetic
Strategies in Chemistry
11.3
silver
ions and moves towards cathode. At
the cathode silver ions is reduced to
silver and
deposits
on the fork. This results in
thin covering of silver on
the cathode (fork).
Battery
e-
Ag
Ag+
Anode
Fork
Cathode
aq.AgNO3
Fig.
11.1 Representation of silver
electroplating
Two
parameters determine the course of
the reaction i) the
deposition current and (ii)
the
cell
potential. Of the two, any
one of them can be controlled as a
function of time
during
the
reaction.
In
a galvanostatic synthesis (Fig.
11.2), a constant current is
applied through the
electrolytic
cell leading to deposits
with good adhesion and a controlled
morphology.
However
the cell potential drifts as
the activity (concentration) of
the reactant is
decreased.
The
drift in the cell potential
may lead to a multiplicity of
products.
A
potentiostatic synthesis is carried
out with a three-electrode
electrolytic cell
(Fig.
11.3).
The synthesis is carried out
by polarizing the electrode to a
desired potential
with
respect
to a reference electrode. The
cell current usually decays
rapidly as the
reaction
proceeds,
both due to low rates of
diffusion of the reactant
molecules from the bulk to
the
electrode
surface as well as due to decrease in
the activity of the
reactant. The
reaction
yields
a pure single-phase product selected
for by the applied
potential.
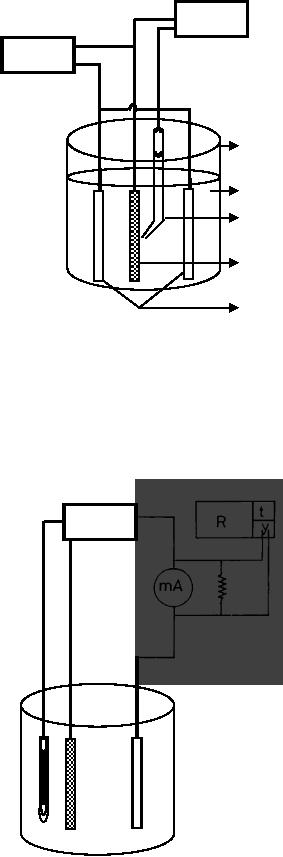
11.4
Electrochemical
Synthesis
V
G
e-
1
2
3
W.E
C.E
Fig.
11.2. Galvanostatic
Synthesis,
G,
galvanostat; V, voltmeter; WE,
working electrode; CE,
counter electrode;1,
electrochemical
cell; 2, electrolyte; 3, Lugin
capillary
P
W.E
C.E
R.E
Fig.
11.3. P, potentiostat; R, recorder;
RE, reference electrode; WE,
working electrode;
CE,
counter electrode
Synthetic
Strategies in Chemistry
11.5
VARIOUS
TECHNIQUES FOR ELECTROCHEMICAL
SYNTHESIS
Various
techniques are employed in
electrosynthesis. A list a few of
them and the nature
of
products obtained from each
are given in Table
11.1.
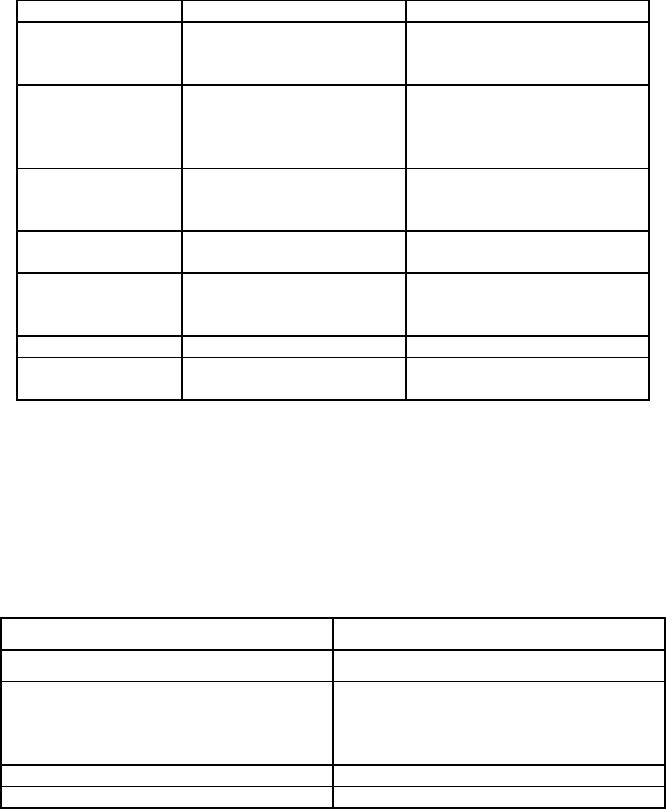
Table
11.1. Summary of the
Electrosynthetic Techniques
Technique
Product
Application
Anodic
oxidation
Coatings/films/powders/
Synthesis
of compounds with
conducting
polymers
high
oxidation state,
corrosion
control,
electrochromism
Cathodic
reduction
Coatings/films/powders
Synthesis
of
electrode
materials
for energy systems,
fabrication
of
hydroxide
films/coatings
Electrolysis
Single
crystals, pure metals
Crystal growth at moderate
of
fused salts, water
and
gases
temperature,
pure
gas
production
Electromigration
Polycrystalline
powders/
Electrode
materials
for
of
reactant species
single
crystals/carbon
batteries
and electrochromism
Layer-by-layer
Synthesis
of composites/solid
Alternate
films/coatings
solutions
voltage/current
synthesis
Electrospraying
Micro
or nanoparticles
Biomedical
applications
Electrospinning
Micro
or nanofibres
Drug
delivery, tissue
engineering,
sensors
During
anodic oxidation a metal ion
in a lower oxidation state or a
monomer like pyrrole
or
aniline is oxidized to a higher
oxidation state or polymer
anodically.
The
anodic
oxidation
technique is especially suited
for the synthesis of
compounds with metal ions
in
unusual
high oxidation states. In
Table 11.2, important anodic
syntheses are listed.
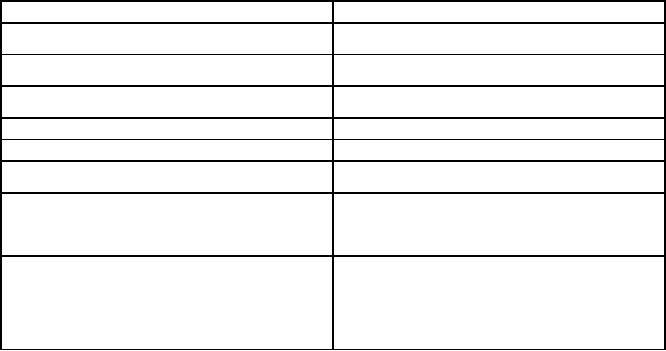
Table
11.2. Oxides and Polymers
Synthesized by Anodic
Oxidation
Compound
Application
Al2O3
Template,
molecular filters
Electrochromic
devices, lithium ion
CoO2.nH2O
batteries,
supercapacitors, and the
protection
film of cathodes in
molten
carbonate
fuel cells
FeO
and MnO2
Electrode
material
Ta2O5
Protective
coating for chemical
equipment,
11.6
Electrochemical
Synthesis
electronic
and sensor devices
Alkaline
water electrolysis
NiO(OH),
Co2O3
Battery,
Organic degradation
PbO2
Magnetic
devices, electrode material
Fe3-xLixO4
WO3 with Co, Cr,
Fe, Mo, Ni, Ru, and
Zn
Electrochromic
devices
RuO2
Supercapacitor
Photocatalyst,
humidity sensor
TiO2
V2O5 nanofibers
Battery
material, active materials
in
electrochromic
and chemochromic devices
Polypyrrole,
polyaniline, polythiophenes,
Sensors,
Electroluminescence, Protective
polyacetylenes,
polyindole
coating,
FET, Organic
semiconductors,
batterries
In
cathodic reduction electric
current is passed through a
metal salt solution and hence
the
metal
is deposited at the cathode.
This
principle is widely used to
obtain metal
coatings.
But
depending upon the
deposition potential, choice of
the anion and the pH of
the
solution
various other reactions take place at
the cathode. Switzer in 1987 introduced
this
technique
for the first time as a
synthetic route to obtain
oriented ceramic films as
well as
Polycrystalline
CeO2 powder was synthesized from
a cerous
polycrystalline
powders.
nitrate
solution. Various oxide
materials are synthesised by
using electrogeneration of
base
by cathodic reduction and their
typical applications are
listed in Table 11.3.
During
electrolysis of fused salts, a
low-melting salt containing
the transition metal
oxide,
is melted and electrolyzed at elevated
temperatures using an inert Pt electrode or
a
reactive
metal electrode such as Fe,
Co, or Ni depending on the
desired product.
During
water
electrolysis pure oxygen and
hydrogen is collected at the
anode and cathode
respectively.
Synthesis
by electromigration, is based on kinetic
control over the reaction
by
electrochemistry.
This technique involves
intercalation or deintercalation of a guest
ion
in
a host lattice by applying an
electric potential between
the electrodes.
During
pulsed electrolysis, the
working electrode is alternately
polarized anodically for
a
length
of time, t1(called
the on-time) and then
cathodically for time, t2
(called
the off-
time).
Voltage
as well as current pulses were
used to obtain oxide films
in the Pb-Tl
Synthetic
Strategies in Chemistry
11.7
system
using a stainless steel electrode and a
mixed Pb-(II)-Tl(I)
bath.
At
low current
densities,
the films were Tl-rich
while at high current densities
the films were
Pb-rich.
Cathodic
deposition of the precursor
film for the YBaCuO
system has been carried
out
from
a mixed metal nitrate bath
containing KCN as well as a
complexing agent.
The
superconductive
films obtained from a
cyanide bath show a Tc ~ 92 K
which is greater
than
all other reported values
for this class of
superconductors, obtained by
electrochemical
techniques.
High
intensity pulsed electric
fields is an interesting
alternative
to traditional techniques like
thermal pasteurization in preservation of
liquid
foods
such as fruit juices or
milk.
Conventional
preservation methods such as
heat
treatment
often fail to produce
microbiologically stable food at the
desired quality
level.
High
intensity pulsed electric
fields processing can deliver safe and
shelf-stable products
with
high nutritional
value.
Pulsed
electrodeposition is an effective method
to prepare
nano
materials of different
morphologies.
Table
3. Oxides Synthesized by
Electrogeneration of Base by Cathodic
Reduction
(reproduced
from [1])
Compound
Application
Gas
sensor, fuel cells
CeO2
La1-xMxCrO3 (M
= Ca, Sr or Ba)
Electronic
conductor
ZrO2
Ionic
conductor
Dielectric
components
BaTiO3
LaFeO3
Oxide
coating
Corrosion-protective
coating
Al2O3, Cr2O3,
Ln2Cr3O12.7H2O
Electrode
material
PbO2
Mo1-xMxO3 (M = Co, Cr, Ni, W or
Zn)
Optical
light modulators
TiO2
Photocatalyst
Nd2CuO4
Superconductor
Optical
and electronic devices
ZnO
Giant
magnetoresistance (GMR)
LaMnO3
WO3
Electrochromic
devices
11.8
Electrochemical
Synthesis
MICRO-
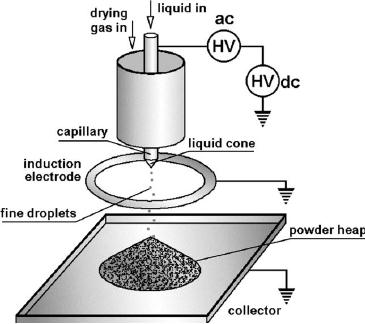
AND NANOPARTICLE PRODUCTION BY ELECTROSPRAYING
Electrospraying
is a
process of liquid atomisation by
electrical forces. It is a
dynamic
process
of droplet generation and simultaneously
charging by means of an electric
field.
Droplets
spraying out of a capillary
nozzle, produced by electrospraying
are highly
charged.
Charged droplets are self
dispersing in space, resulting in
the absence of
droplet
coagulation.
The deposition efficiency of a charged
spray on an object is higher
than for
an
un-charged spray. This
feature can be advantageous in thin-film
formation or in
surface
coating. Generation of the
droplet and its size can be controlled by
controlling
the
flow rate of the liquid and
the voltage at the nozzle.
Nearly monodisperse size
distribution
is achievable.
The
size of the electrospray droplets can
range from
micrometers
to nanometers.
Fig.
11.4. Schematic for
production of particles of uniform size
by electrospraying
(reproduced
from [2])
Electrospraying
technique is a single-step, low-energy,
and low-cost material processing
technology,
which can deliver products of
unique properties.
Electrospraying
is
exploited
in many industrial processes
such as painting, fine
powder production, or
micro-
and nanothin film deposition. It is also
employed in microfluidic devices
and
nanotechnology.
Spraying
solutions or suspensions allows
production of particles,
Synthetic
Strategies in Chemistry
11.9
ranging
from micro to nanometer
size.
Production
of particles of uniform size can
be
accomplished
by using pulsed or ac superimposed on dc
bias voltage for liquid
jet
excitation.
By
tuning the frequency of ac
voltage, uniform size of droplets
are formed
by
disintegrating the liquid
jet. Various parameters can control
the droplet size they
are
the
bias and ac voltage magnitudes, ac
voltage frequency, and volume
flow rate of the
liquid.
Uniform
droplets can be achieved when ac and dc
voltages are adjusted
such
that
the droplets are formed and
detached at the voltage
peaks.
A
schematic diagram of
a
system for harmonic spraying
is shown in Fig.
11.4.
Electrospraying
is exploited for the
generation of micro/nanospheres for
biomedical
applications
since the process has an advantage of
not making use of any
external
dispersion/emulsion
phase which often involves
ingredients that are
undesirable for
biomedical
applications.
Chitosan
micro/nanospheres
were
synthesized
by
electrospraying
with acetic acid solution is exploited
for drug delivery
applications. This
technique
is also used to prepare polycaprolactone
(PCL) polymer particles with
a
different
microstructure by the evaporation of
solvents during the
electrospraying
process.
MICRO-
AND NANOFIBRES PRODUCTION BY
ELECTROSPINNING
Electrospinning
is a
process in which a high
voltage electric field is
applied to a melt or
polymer
solution in order to attain charge
repulsion on the liquid
surface. This overcomes
surface
tension there by a thin
liquid jet is ejected. Narrow
jet diameter is attained due
to
the
electrostatic repulsion caused
between the charges on the
liquid surface and
the
collector
that has a different
electric field. Solid or
polymer fibres ranging from
10 m to
10
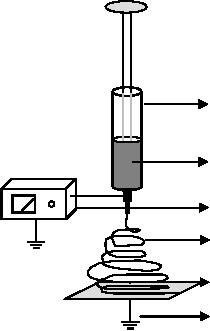
nm is attainable. As shown in Fig.
9.5, a typical electrospinning setup
consists of a
syringe
with a capillary nozzle
through which the liquid to
be electrospun is forced; a
high
voltage source with positive or
negative polarity to charge the
liquid jet and a
ground
collector.
Electrospun
textiles are exploited in
preparing filters, semi-permeable membranes,
as
scaffolding
for tissue engineering and in drug
delivery. Electrospinning has
flexibility in
selecting
materials for drug delivery
applications.
Either
biodegradable or non-
degradable
materials can be used to control
whether drug release occurs
via diffusion
alone
or diffusion and scaffold degradation.
Due to the flexibility in
material selection a
11.10
Electrochemical
Synthesis
number
of drugs can be delivered including:
antibiotics, anticancer drugs,
proteins, and
DNA.
Syringe
Sample
Voltage
generator
(
Non-woven
fiber
Collector
Ground
Fig.
11.5. Schematic of an electrospinning
system
CONCLUSION
Electrochemical
techniques such as anodic
oxidation, cathodic reduction,
alternating
current
pulsing, electrospraying and
electrospinning provide simple, cost
effective
alternative
routes for the production of
micro or nanomaterials, thin
films, coating,
composites
having unique properties and
applications. It is hoped that the
ease and
versatility
of this technique will find will
find it a permanent place in synthetic
chemistry.
REFERENCES
1.
G. H. A. Therese and P. V. Kamath, Chem.
Mater., 12
(2000) 1195.
2.
A. Jaworek, Powder
Technol., 176
(2007) 18.
3.
A. Jaworek, A.T. Sobczyk J.
Electrosta., 66
(2008) 197.
4.
N. Arya, S. Chakraborty, N. Dube,
D.S Katti, J Biomed
Mater Res B Appl
Biomater.,
2008 (In
Press)
5.
S. Tan, X. Feng, B. Zhao, Y. Zou, X.
Huang, Mater.
Lett., 62
(2008) 2419.
6.
T. J. Sill, H. A. von Recum, Biomaterials,
29
(2008) 1989.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES