 |
CHAPTER
- 10
THE
ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY
And he
said unto them, it is not
for you to know the
times
or
the seasons, which
the
Father
hath put in his own
power.
The Holy
Bible (The
Acts 1:7)
My
times
are in
thy hand.
The
Holy Bible (Psalm
1:15)
To
every thing there is a
season, and a time
to
every purpose under the
heaven:
A
time to be born, and a time
to
die; a time
to
plant, and a time
to
pluck that which is
planted;
A
time
to
kill, and a time
to
heal; a time
to
breakdown, and a time
to
build up;
A
time
to weep,
and a time
to
laugh; a time
to
mourn, and a time
to
dance;
A
time
to
cast away stones, and a time
to
gather stones together; a time
to
embrace, and a
time
to
refrain from
embracing;
A
time
to
get, and a time to lost; a time
to
keep, and a time
to
cast away;
A
time
to
rend, and a time
to
sew; a time
to
keep silence, and a time
to
speak;
A
time
to
love, and a time
to
hate; a time
of
war, and a time
of
peace.
The
Holy Bible (Ecclesiastes
3:1-8)
Noah's
Ark The Technological
Marvel
13.
And God said unto
Noah, The end of all flesh
is come before me; for the
earth is
filled
with violence through them;
and, behold I will destroy
them with the
earth.
14.
Make thee an ark of gopher
wood; rooms shalt thou make
in the ark, and shalt
pitch
it
within and without with
pitch.
15.
And this is the fashion
which thou shalt make it of:
The length of the ark
shall be
three
hundred cubits, the breadth
of it fifty cubits, and the
height of it thirty
cubits.
16.
A window shalt thou make to
the ark, and in a cubit
shalt thou finish it above;
and
the
door of the ark shalt
thou set in the side
thereof; with lover, second,
and third stories
shalt
thou make it.
Genesis
6: 13-16, The
Holy Bible
10.2
Role
of Synthesis in Materials
Technology
What
is new in Materials
Technology?
The
Living Cell is the All Time
Marvel of Almighty God, The
Creator. The Living
Cell
can
apparently handle enormous
number of unimaginable, uncomprehendable
and
difficult
problems (functions) with
ease and spontaneity.
The
Living Cell performs
multiple
functions (reproduction, growth, defense,
protein synthesis, transport
of
nutrients,
information storage, site directed
information transfer, communication,
energy
conversion
and energy storage, sensing)
simultaneously. All vital functions
for the
sustenance
of life takes place in the
living cells. Thus the
Living Cells are
self-
replicating,
self-containing and
self-maintaining.
One
of the goals of Materials
Technology
is to design and synthesize the material
with artificial intelligence
that
replicate
Living Cell in all
aspects.
An
inquisitive mind now poses
the following
questions:
What
is the Living Cell? What
does a Cell mean? Where does
the term `Cell'
originate
from?
How can the Living
Cell be multifunctional and
versatile? How cells
form
complex
organisms? What is the
structure of the Cell? What
are the dimensions of
the
Living
Cell? What are the
constituents of the Cell?
Can the Living Cells be
mimicked?
Can
such mimics of the Living
Cells act as molecular
machines and
revolutionize
Materials
Technology? The
queries are recurring.
The
Cell is the basis of life.
The Cell is the smallest
unit of all living
organisms
whether
it be unicellular (eg., bacteria) or
multicellular (eg., human
beings). Human
beings
have an estimate of 100 trillion
(1014)
cells. A typical cell is of 10 �m size
and 1
nanogram
mass [1].
Robert
Hooke is the originator of
the term "Cell" and has
been derived from the
latin
word
`Cellula' meaning `a small
room'. It is worth knowing
few good things about
Robert
Hooke which are depicted in
the next few
lines.
Robert
Hooke (18th July, 1635 - 3rd
March
1703)
Robert
Hooke was a remarkably industrious
scientist and philosopher. He was an
active,
restless,
indefatigable genius even
almost to the last days of
his life. One of the
important
contributions
of Robert Hooke to Biology is
his book "Micrographia"
published in 1665
[Fig.
10.1 (a)]. Robert Hooke
coined the term "Cells".
This has originated from
his
microscopical
observation of thin slices of cork
(tissue of light soft bark
of Mediterranean
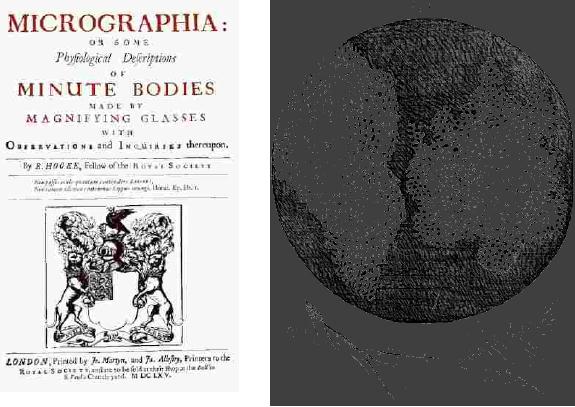
Synthetic
Strategies in Chemistry
10.3
tree).
He coined the word `Cell' to
the pores separated by walls
because the
observation
reminded
him of the cell of a
monastery (small rooms where
monks lived in) [Fig.
10.1
(b)].
He
has recorded his study of
the plant tissue in "Observation XVIII"
of the
Micrographia
as follows [2]:
"I
could exceedingly, plainly
perceive it to be all perforated and
porous, much like a
honey-comb,
but that the pores, or
cells, ........ were indeed the
first microscopical
pores
I
ever saw, and perhaps, that
were ever seen, for I had
not met with any
Writer or Person,
that
had made any mention of them
before this ....".
Truly,
history owes to this industrious
scientist and philosopher.
(a)
(b)
Fig.
10.1. (a) Title page of
Micrographia (1665) [3], (b)
Robert Hooke's drawings of
the
cellular
structure of cork (plant
tissue) and a spring of sensitive
plant from
Micrographia
[4].
Living
cells are divided into
two types, namely, the
Procaryotic Cells and
the
Eucaryotic
Cells. Procaryotic cells
possess simple structure
(eg. Bacteria cell) [5].
Cells
10.4
Role
of Synthesis in Materials
Technology
without
a nucleus were grouped
together as "Prokaryotes'. We human
beings, and most
of
the animals and plants,
belongs to the life form
enjoying cell nucleus and
are
collectively
called Eukaryotes [6].
Eucaryotic cell (they carry
their DNA wrapped in a
cell
nucleus) possesses complex
structure as represented in Fig.
10.2. The cells
are
surrounded
by a double layer membrane. In
many cases the cells
are further shielded
by
a
cell wall. As a result
chemical processes taking place in a
cell would not easily
get
disturbed
by the surrounding
environment.
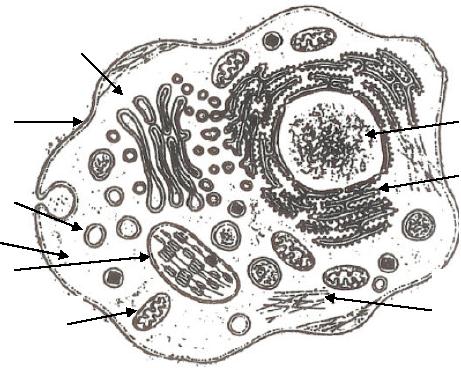
Golgi
Apparatus
Cell
Nucleus
Membrane
Rough
Lysosome
Endoplasmic
Reticulum
Cytoplasm
Chloroplast
Smooth
Endoplasmic
Mitochondria
Reticulum
Fig.
10.2. Schematic representation of a
eukaryotic cell and its
compartments [6]
The
cell possesses secluded areas
for specific
functions.
The
major organelles
(specialized
subunit with in a cell that
has a specific function to
perform) and cellular
structure
include the nucleus, ribosomes,
mitochondria, golgi apparatus, cytoplasm,
rough
endoplasmic
reticulum and smooth endoplasmic
reticulum. As houses are
divided into
living
room, dining room, kitchen
and bed room, living cells
too are
compartmentalized
as
shown in Fig. 10.2. Each
compartment has a specific
set of functional tasks.
For
instance,
the nucleus contains most of
the DNA which is the carrier
of genetic
information
in all the cellular life
forms. The mitochondria are
the power houses of
the
Synthetic
Strategies in Chemistry
10.5
cells.
These are the sites where
cellular respiration and a consequent
release of chemical
energy
from food takes place. Ribosomes
are the protein synthesizing
machines of the
cell.
Ribosomes are the cells
protein factory.
Can
we imagine a data storage
device of micrometer
(10-6 m) size but can
squeez
the
data equivalent of five
high-density floppy disks (5 x
1.44 MB = 7.2 MB) ?
Yes,
such a data device is
existing in Nature. The
chromosome, a very long
stretch of
DNA
wound up in a complicated way,
which determines the genetic
identity of every
living
organism on this plant is an
example of such data storing
device.
Can
we imagine a motor that is
running on and on and on but
only of size
measuring
a few hundredths of a thousandth of a
millimeter?
For
your surprise the motor is
already in existence. It is a system
mainly consisting of
the
proteins
actin and myosin. The system
serves to power our muscles.
Actin is a soluble
protein
found in muscle cells. It is
the main component of the
thin filaments. Myosin
is
the
motor protein that generates
the force and movement in
contraction of muscles.
Myosin
is the oxygen storage
protein of the muscle. When
Myosin carries an oxygen
molecule,
the oxygen molecule is
deeply burried within the
structure of the
protein.
Because
of the weak interations present
the protein is capable of
rearranging its
structure
to
be able to take up oxygen or to set it
free by way of rearranging
itself so that a tunnel
is
opened
between the oxygen binding
site and the rest of the world.
For such rapid
rearrangments
to take place weak interactions should be
present with in the
protein
structure
as well as with the substrate.
The following are some of
the weak interactions
found
in biological systems (The
Cell).
i.
Hydrogen bonding: Hydrogen
atom is normally bound to
just one atom of oxygen
or
nitrogen.
In Hydrogen bonding the
Hydrogen atom starts interacting
with a second atom.
Hydrogen
bonding is responsible for
the extremely high boiling
point in spite of its
modest
molecular weight. If hydrogen
bonding did not exist,
water would be a gas
at
ambient
temperatures.
ii.
Salt bridges: This
is because of the electrostatic
attraction between parts of
molecules
having
opposite electrical charges.
iii.
Van der Waals forces: These
forces exist between the
negatively-charged cloud of
electrons
of one atom and the positively-charged
nucleus of the other.
10.6
Role
of Synthesis in Materials
Technology
iv.
The Hydrophobic interaction: The
tendency of oily, water-avoiding
molecular
surfaces
to stick together and shut
out any water
molecules.
Thus
the first lesson one need to
learn is that the key to
generate materials that
replicate
living
cells or biological systems lies in
designing synthetic strategies
based on weak
interations
between the reacting systems as
represented pictorially in Fig.
10.3..
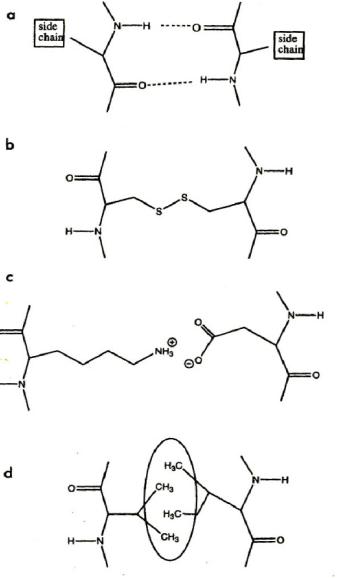
Fig.
10.3. Interactions that
stabilize the local
structures in proteins: (a)
Hydrogen bonds
(secondary
structure), (b) disulfide bridges
(tertiary or even Intermolecular,
(c) salt
bridges,
(d) hydrophobic interaction.
The oval shape symbolizes
the hydrophobic area
from
which water is excluded
[6].
Synthetic
Strategies in Chemistry
10.7
Can
we imagine a catalyst capable of
converting the inert
nitrogen gas from the
air
into
nitrogen fertilizer at room
temperature and atmospheric
pressure?
The
enzyme nitrogenase present in nodule
bacteria that live in symbiosis
with certain
plants
and provide them with
freshly made nitrogen
fertilizer produced from air
and
water.
There is no technical catalyst
producing ammonia from
elemental hydrogen and
nitrogen
at ambient temperature and pressure as
the nitrogenase of nodule bacteria.
Thus
it
is the dream not yet
realized by the scientists
all over the
world.
Can
we dream of the synthesis of
natural products with 100 % enantiomeric
excess
(ee)?
In
organic chemistry, particularly in
the synthesis of natural
products, one of the
major
issues
of concern even today is to
synthesize exclusively one of the
two mirror-imaged
structures
of chiral compounds. Literally,
synthetic chemists are
battling for every
single
digit
enhancement in the value of ee
beyond the statistically
50%. Interestingly
enzymes
can
distinguish the mirror
images with 100%
reliability.
Can
we synthesize catalysts
(nonnatural)
which are on a par with
natural enzymes in their
performance? This is
yet
another
challenge facing materials
technology today.
Other
important challenges ahead in
Materials Technology are
achieving computer
technology
that matches with human
brain, achieving telecommunications
technology
that
matches with nervous system of
human body. The only
means of realizing
such
breakthroughs
in materials technology is through
the evolving synthetic
strategies in
Chemistry.
Some
Benefits from Materials
Technology
Audio
tapes, audio tape players,
audio tape recorders, calculators,
cameras, compact disks
(CD's),
CD players, Barcode, Colour Printers,
Computers, Digital video
disk (DVD),
Electronic
commerce, Electronic data
interchange, E-mail, Internet,
Fax machines,
Laboratory
equipment, Laser printers, Laser
pointers, Liquid crystal
display, LCD,
Mailing
services, Measuring instruments, Modem,
Network/cable television,
Periodicals,
News
papers, Over head
projectors, Photocopy machines,
Play ground equipment,
Radio,
Refrigerators,
Slide Projectors, Scanner,
Search Engines, Switching
technology,
Telephones,
Transparencies, Type writers,
Video cameras, Vedio
conferencing are all
some
of the marvels of Materials
Technology [7].
10.8
Role
of Synthesis in Materials
Technology
Materials
Technology Vs Material's
Technology
Materials
technology is a science and a knowledge
area that describes
properties,
functions
and applications of different materials
[8]. Materials technology is
dependent
on
Material's technology, i.e.
the way materials are
architectured or build or
constructed
or
fabricated or synthesized. To gain
knowledge of Materials technology one
should
understand
the role of synthesis in
generating the materials.
New materials,
improved
production
techniques, and miniaturization are
the three essential ingredients
that have
tremendous
potential to trigger major
technological revolutions.
Role
of Synthesis
Materials
synthesis is so vital that
the early history of man
kind (from the stone age to
the
iron
age) is classified based on
the materials that started
new eras.
So
far the
classification
of eras is associated with or
based on materials that have
revolutionized the
respective
eras.
But
the role of synthesis has
now become so crucial in
materials
technology
that the production methods
are going to be used as
milestones of
development
rather than the material
itself. Initially materials
that are either
simply
found
or gathered from nature (wood, bones,
stones) served mankind. Progress has
been
made
from such naturally existing
materials to the use of
metals retrieved from ores
using
fire.
Metals were found to be more
versatile in their use
rather than naturally
occurring
materials.
Currently,
metals, glass and other
materials are being replaced by
polymers. This age
which
we live can be truly regarded as
polymer age because the
survival of mankind
becomes
questionable with out
polymers (substances that
are chemically assembled
from
smaller
molecules).
Again
polymers are more versatile
than metals and
naturally
occurring
materials like wood or stone.
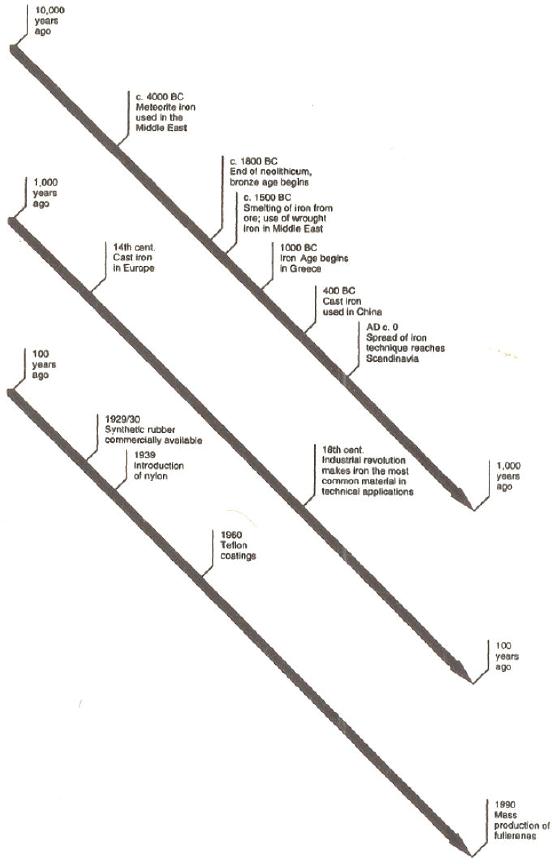
Milestones in the use of
materials during the
last
10,
000 years have been
illustrated in Fig. 10.
4.
But
still we have not our
destination in technological advancement.
Even though
polymers
have the potential to cater to
many of our needs they can
not fulfill all
the
demands
we have for advanced materials.
The problems associated with
polymers are:
valuable
resources and energy are
used up in the production of
polymers. Also they
are
neither
biodegradable like wood nor
recyclable like iron.
Synthetic
Strategies in Chemistry
10.9
Fig.
10.4. Time line illustrating
the use of materials during
the past 10, 000 years
[6].
10.10
Role
of Synthesis in Materials
Technology
Even
though polymers have
revolutionized the way we
live, in comparison to
biological
materials
they are far inferior as
they in no way contain any
significant amount of
information,
also they cannot store energy, or
they cannot act in an intelligent
way. Thus
the
focus of the evolving
synthetic strategies has
been to produce materials
mimicking
biological
materials. Such materials
should be functional and adaptive on a
molecular
scale.
Like
our fore fathers and
ancestors we may be of the
opinion that our
present
technology
is modern, advanced, accomplished and
ultimate. Also we may tend
to
believe
that only incremental
improvements can be brought about in
the present day
technology.
Major technological breakthroughs
are believed to be hardly possible.
But
this
is not true. There is large
room for the change to
betterment as change happens to be
the
law of life. The enormous
scope and potential for such
a major advancement can be
noticed
if we compare our present day synthetic
strategies or production
methodologies
with
the synthetic strategies
adopted by molecular machinery in
living cells. So long
almost
all the available synthetic
strategies handled bulk
amounts (countless atoms or
molecules)
which yielded materials
whose properties can only be
controlled with in a
limit.
But the properties of
materials obtained by adopting
such synthetic
strategies
dealing
with bulk amounts are
not adaptive or intelligent.
Even though the tools
and
machinery
that one currently uses to
carry out simple processes
automatically they can no
way
go down below macroscopic or at
the most microscopic levels.
In sharp contrast, in
the
living cells, the structures
of inorganic building materials
are controlled at the
molecular
level guiding the
precipitation of the mineral
from solution for
instance.
Incidentally,
the most complex machines of
the cell are not
bigger than 25 50 nm.
Thus
there
is ample scope and also the
way is long to meet the
materials technology
mimicking
the
Living Cell. At the moment
The Time of reaching the
destination is not
clear.
Information
technology, Nanotechnology and
Biotechnology are regarded as
the three
eyes
of the new millennium. New
synthetic strategies can open access to
new materials.
Materials
are transformed into useful
products by working on them
with hands and tools.
Production
technology underwent a drastic change.
From millennia useful
products are
made
manually. Now most of them
are made by machines. Except
those processes
where
decision-making is involved all
others are being carried
out by machines. As a
Synthetic
Strategies in Chemistry
10.11
consequence
length scale is no longer
limited to such parts that
human workers could
grasp
with their hands. Thus
miniaturization of production has paved
the way for
the
miniaturization
of the products.
INFORMATION
TECHNOLOGY:
The
Beginning of Information Technology
The Age of the Printed
Book:
Now
this very second you
are reading this book. It
means you are getting
benefited by
the
revolution of book publishing
technology that has started in
15th century. Johannes
Gutenberg
(1397 1468) conceived
the idea of printing books
with movable type. He
developed
his idea into a working
technology. It is no easy job to
achieve uniform
dimensions
of letters. Such sufficiently
uniform dimensions of letter
type facilitates a
flat
printing
surface. Hence smooth lines
are obtained.
Through
years of persistent
hard
work
Johannes Gutenberg could
overcome this problem by
making the type from
metal.
He
could cast all of them in
the same mold, which
could be combined with
different
letter
shapes and adjusted in its
width. This development is a
result of several years of
hard
work. To achieve this
objective he has to borrow considerable
amounts of money.
He
could not have given
the world the printing
technology if he were not to
be
knowledgeable
in metallurgy. His major
success involves producing
160-170 copies of
the
42-line Bible shown in Fig.
10.5. The name 42-line Bible
refers to the number
of
lines
of print on each page. This
work of Johannes Gutenberg is of
iconic status as this
marked
the beginning of `Gutenberg
Revolution' and `the Age of
the Printed Book'.
The
printing of 42-line Bible by
Gutenberg is a remarkable development in
the history of
mankind
as this has broken the
information monopoly. The
new technology has put
an
end
to the Dark Ages. It has
brought about the
reformation, the enlightenment and
the
rise
of modern science. Interestingly, with in
a span of 50 years after the
production of
Bible
with printing technology
developed by Johannes Gutenberg,
more books were
produced
than in the 1000 years before.
The technology Johannes
Gutenberg developed
lasted
for more than 500 years.
Later on this printing
technology of Gutenberg was
replaced
by light reprography. This is
followed by the new
information revolution
brought
about with the invention of
personal computers.

10.12
Role
of Synthesis in Materials
Technology
Fig.
10. 5. (a) A copy of the
42-line Holy Bible, the
major work of Johannes
Gutenberg
[9].
Advent
of Computers and Internet
Information Explosion:
Advent
of computers and widespread growth of
availability and use in internet
has
brought
about revolution in the
field of information technology. Since
this development
has
only taken place over the
past two decades we are
fortunate enough to witness
the
progress.
But how could this
happen? What is the driving
force for such a
drastic
explosion
in information technology? Literally
with computers and internet
the whole
world
is at our finger tips. The
marvelous progress can be attributed to
the new synthetic
strategies
that facilitated miniaturization of
electronic elements and
circuits.
Miniaturization
means not alone saving
space or materials but
miniaturization of
electronic
circuits made them cost
effective, efficient and faster. 1000
fold enhancement
is
memory capabilities and calculation
speeds have been achieved
with in a short span
of
10
years i.e., from early
1980's to 1990's. The number
of transistors in typical
microchip
kept
on increasing exponentially from
1971.
For
every 18 months the number
of
transistors
on a microchip doubled.
In
1971 the microchip contained
only 2300
transistors
when Intel launched the
world's first
microchip.
Nearly
a hundred fold
increase
in the number of transistors
has been achieved by 1985
with the creation of
Intel
386
processor with 2, 75, 000
transistors. And today the
Tukwila contained 2
billions
transistors.
Very recently, i.e., on
14th April 2008, Intel,
the microchip leader,
unveiled
Synthetic
Strategies in Chemistry
10.13
two
drastically different processors.
They succeeded in creating
the worlds smallest
microchip
(computer processor) namely the
atom chip as well as the
worlds biggest chip
namely
the Tukwila. The size of the
atom chip is of a baby's
finger nail. The chip
packs
47
million transistors. But consumes
only over a half of a watt
of power. The atom
chip
can
power hand held internet
devices namely the mobile
internet devices, small hand
held
computers
that will help in emailing,
letter writing and calculations. On
the contrary the
Tukwila,
the new Intel chip
possessing the highest
number of transistors ever
put on the
slab
of silicon with 2 billion
transistors can do the work of
four computers. This
world's
biggest
microchip consumes 130-170 watts of
power. This will help
scientists to build
gaint
`number crunchers' far more
powerful than the Tata-CRL
`Eka' super computer
built
in India (the Tata-CRL
`Eka', computer is the
world's forth-fastest
computing
machine).
With the help of such super
computers fuelled by microchips
with 2 billion
transistors
complete genetic simulation of a
human cell can be achieved
with in a span of
a
decade or even lesser time
period. This facilitates
doctors to precisely simulate
an
unhealthy
cell in a human. Thus exact
medication can be possible. With
the advent of
nanotechnology
unimaginable advancements in the field of
information technology
are
anticipated.
NANOTECHNOLOGY:
The
word Nanotechnology was used
for the first time by
Taniguchi at the University
of
Tokyo,
Japan, in 1974. He was then
referring to need of electronics
industry to engineer
materials
at nanometer scale
[10].
On
29th of
December, 1959, Richard P.
Feynman took the shiny
example of the
living
cell to drive home his
point that individual atoms can be
arranged in the way we
want
them to be.
With
this ultimate degree of
miniaturization all the
information
contained
in all the books in the
world can be stored in the grain of a
sand. Living cell is
not
only capable of storing
enormous amount of information in a
very small volume
but
also
equipped with the hard ware
to read out the information
and retrieve the same
when
needed
and put the same into
action. In an analogous way
Richard P. Feynman
professed
that
it should be possible to write the
entire Encyclopaedia Britannica
onto the point of a
needle.
In those days when computers were
huge machines the wiring of
which filled the
10.14
Role
of Synthesis in Materials
Technology
whole
room completely, he is genius
enough and fore sighted to profess
that computers
of
the future should be made of
wires that would only be 10
or 100 atoms in diameter.
Role
of Synthesis in Nanotechnology:
Carbon
materials are very important
for Nanotechnology to flourish.
Carbon materials
are
vital, versatile, amazing and
unique. Carbon exists in
different allotropic
forms,
namely,
graphite, diamond, fullerene, and
nanotube. Graphite with its
two dimentional
hexagonal
array of carbon atoms and diamond
with its three dimensional
structure are
well
known. Fullerene and nanotubes
are newly discovered allotropic
forms of carbon.
Synthetic
Strategy that lead to the
formation of Fullerenes:
A
dream team of five scientists namely
Kroto, Heath, O'Brien, Curl
and Smally in Rice
Quantum
Institute, Texas were trying
to understand how long-chain
carbon molecules
(cyclopolyynes)
are formed in interstellar
space.
To
find an answer, they
started
vapourizing
graphite, the grand old
allotrope of carbon, by irradiating
with laser. This
experiment
has lead to the serendipitous
discovery of C60 molecule, named as
Buckminsterfullerene.
The structure of C60 molecule consisted of 32 faces, 12 of
which
are
pentagonal and the remaining 20
are hexagonal. The structure
of C60 is analogous to
common
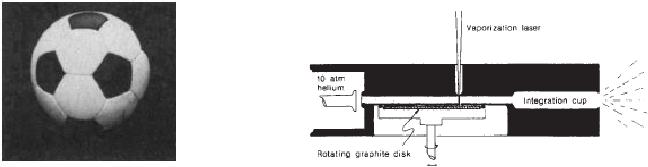
foot ball shown in Fig.
10. 6. (a) The experimental
set up containing the
vapourization
chamber is shown in Fig. 10.
6. (b). Later on many studies
have been
carried
out on the properties and applications of
fullerens and fullerene derivatives.
Not
oniy
that, fullerene research paved
path for the discovery of
multiwalled carbon
nanotubes
by Ijima. Simply the
synthetic methodology adopted
has changed the course
and
destination of carbon science and
technology.
Carbon
species from the surface of
a solid graphite disk are
vapourized using a
pulsed
laser
source in the presence of He environment.
Nd:YAG laser producing pulse
energies
of
~ 30 mJ is used. In a typical experiment
the pulsed valve is opened.
Vapourization
laser
is fired onto the rotating
graphite disk. Carbon
species start vaporizing
into the
helium
stream, cooled and partially equilibrated
in the expansion. As a result
molecular
beam
is formed which travels into
the ionization region. The
clusters were ionized by
a
laser
pulse and the products were
analysed by mass spectrometer.
During the process
graphite
disk is rotated slowly to
produce a smooth vaporization
surface.
The
vaporization
laser beam is focused through
the nozzle to strike the
graphite. The species
Synthetic
Strategies in Chemistry
10.15
in
the vaporized graphite plasma
are cooled and clustered by
the thermalizing
collisions
of
He carrier gas. Also the
carrier gas provides
necessary wind to carry the
cluster
through
the remaining of the nozzle.
The cluster filled gas
expands freely at the
nozzle
and
form a supersonic beam which
is probed by mass spectrometer [11].
Kroto, Smalley
and
Curl were awarded nobel
prize for Chemistry in 1996
for their discovery
of
Fullerenes
in 1985.
Fig.
10.6. (a) A football (the
C60 molecule is supposed to have
the structure formed
when
each
vertex on the seams of such
a ball is replaced by carbon atm,
(b) Schematic diagram
of
the pulsed supersonic nozzle
used to generate carbon
cluster beams
Even
though Fullerens were
discovered in 1985, it is not
until 1991 when Kratschmer
and
Huffman
evolved a synthetic strategy
based on arc discharge for the
mass production of
fullerenes
that fullerene research grew
rapidly and in one way this is a
foundation stone
for
the future discovery of
nanotubes by Ijima in 1991.
Hence synthetic strategies
play a
pivotal
role in the birth as well as
destination of any new
technology.
With
the ways being available
for the mass production of
Fullerenes, derivatives of
fullerenes
could be synthesized.
Endohedral
compounds, exohedral compounds
and
heterofullerenes
are the three classes of
fullerene derivative
[12].
Fullerene
derivatives with atleast one atom or
ion located inside the 7 �
cage of
fullerene
are called endohedral
fullerenes.
Endohedral
compounds are more
polar
compared
to the parent fullerene
making the separation of fullerenes
easier. Electronic
properties
of fullerenes can be tuned by forming
endohedral derivatives.
Endohedral
compounds
find utility in the
fabrication of solar cells,
linear optical units and in
photo
conductors.
Some of the ways of
synthesizing endohedral derivatives
are by heating the
fullerene
directly with the guest gas
under pressure; evaporating fullerene in
presence of
10.16
Role
of Synthesis in Materials
Technology
metals
or metal oxide to be hosted by using
laser source or by generating an
arc.
Endohedral
compounds with noble gases,
alkali metals and lanthanides
are well known.
Recently
N@C60 has also been synthesized.
La@C82
could be
synthesized by evaporating
graphite
impregnated with La2O3 using a laser.
Rg@C60
(Rg-rare
gas), Li@C60
have
also
been
synthesized [13].
Exohedral
compounds: By
reacting C60 with CrO2(NO3)2 a
compound C60Cr2N6O21 was
obtained.
In this compound 50% Cr
species are in Cr3+ and the other 50%
were found to
be
in Cr6+ state. Such exohedral
compounds are useful for
catalytic and medicinal
applications.
Intercalation
Compounds: Alkalimetal
intercalated fullerene compounds
were found to
be
super conductors. For instance,
K3C60
has a
transition temperature of 19.8 K
and
Cs3C60
exhibited a
transition temperatures upto 40 K.
Compounds like (NH3)6Na3C60
(NH3)8Na2C60
showed
improvements in transition temperatures.
Ba6C60
and Sm3C60
have
also
been found to be
superconductors.
Carbon
Nanotubes
Synthesis
of carbon nanotubes with
desired properties in large quantities is
a challenge
ahead.
The second problem in the
successful utilization of CNT's
for a variety of
Technologies
is to remove the impurities present
such as the catalyst
nanoparticles (Fe,
Co,
Ni-Y and few others), amorphous
carbon and fullerenes. The
third hurdle is to
develop
synthetic strategies that
exclusively give either
SWNT's or MWNT's as
currently
there is no path available to
separate them. Making them
soluble is also a note
worthy
problem.
Carbon
nanotubes are a key
component of nanotechnology. Carbon
nanotubes
(multiwalled
tubes) were discovered by the
Japanese scientist Iijima in
1991. Carbon
nanotubes
were obtained as a by-product
during fullerene synthesis.
Discoovery of
carbon
tubes has supplemented the
fullerene research to a major
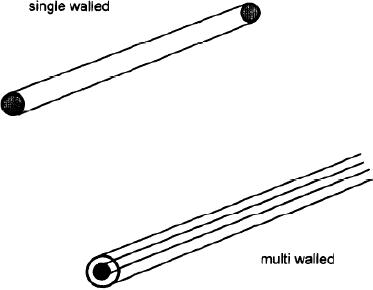
extent. Multiwalled
carbon
nanotubes should be distinguished
from single walled carbon
nanotubes. The
difference
between them is pictorically
represented in Fig. 10. 7.
Single walled carbons
nanotubes
contain one single cylinder
whose wall is made up of
hexagonal carbon
structure.
Multiwalled carbon nanotube
contains concentric cylinders
(one inside the
other)
with each cylindrical wall
being made of a graphene sheet.
Synthetic
Strategies in Chemistry
10.17
Fig.
10.7. Schematic representation of
single walled and multiwalled
carbon nanotubes
[12]
The
discovery of single walled
carbon nanotubes (SWCNT's) is
incidental. A series of
failed
attempts to synthesize MWCNT's
(multiwalled carbon nanotube's)
filled with
transition
metals resulted in the
formation of single walled
carbon nanotubes. The
credit
of
the discovery of single
walled carbon nanotubes
should be given to two
research
groups
who worked independently,
namely Iijima and Ichihashi,
NEC (Nippon Electric
Company),
Japan as well as Bethune et
al., from IBM, California
[14].
Nanotubes
of carbon are not soluble.
As a result separation and purification
are a
problem.
This has hindered the
large scale production of
carbon naotubes partially.
Fortunately
these tubes are less
susceptible to combustion. So heating in
oxygen can
brun
the impurities (other forms
of carbon namely amorphous
carbon) and can yield
pure
CNT's
[13].
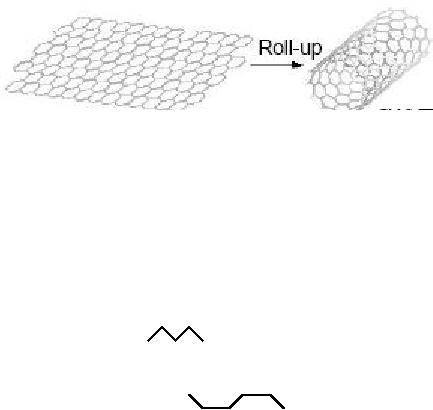
Single
walled carbon nanotubes can be envisaged
or conceptualized or understood as
seamless
(continuous and uniform through
out) cylinders rolled up
from graphene sheet
as
represented in Fig. 10. 8. A graphene
sheet is a monolayer of sp2 bonded carbon
atoms.
10.18
Role
of Synthesis in Materials
Technology
Single
walled nano tube
Graphene
Sheet
Fig.
10.8. Hexagonal net work of
carbon atoms rolled upto make a
seamless cylinder
[15].
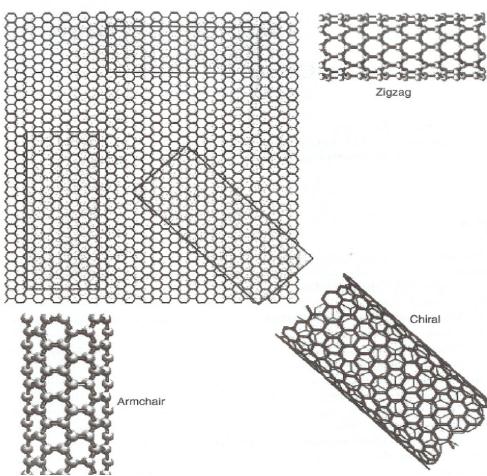
Depending
on the way in which the
graphene sheet is rolled up there can be
three types
of
carbon nanotubes, namely,
zigzag, armchair and chiral. In
zigzag tubes some of the
C-
C
bonds lie parallel to the
tube axis. The name zigzag
comes from the fact
that the edge
of
the tube possesses zigzag
structure (
).
In armchair tube some of the
C-C bonds
lie
perpendicular to the tube
axis. The name armchair
comes from the very
shape of the
edge
of the tube which looks
like arm chair (
).
Intermediate
orientations of
the
graphene sheet result in `chiral'
carbon nanotubes.
Thus
the classification of
nanotubes
into zigzag or arm chair is
based on the appearance of
the rim of the
tube
formed.
The formation of the three
types of nanotubes by changing
the way of rolling
the
graphene
sheet is represented schematically in
Fig. 10.9
Cabon
nanotubes have several
potential applications. This is
because of their
unique
properties.
Due to the high mechanical
stability carbon nanotubes
are now being used
in
carbon-carbon
composites. As carbon nanotubes are
capable of emitting electrons
from
the
tube ends they are
used for flat screen
applications. Carbon nanotues serve as
host
materials
for Li or H2 and can be exploited in energy
storage applications.
Synthetic
Strategies in Chemistry
10.19
Fig.
10. 9. Schematic representation of
the relation between
nanotube and graphene [16].
Evolution
in Synthetic Strategies
Some
of the ways of synthesizing
nanotubes include:
1.
Arch-discharge or vaporization process
(in the presence of
transition metal
catalyst)
2.
Laser-evaporation of graphite (Laser
furnace process)
3.
Chemical Vapour Deposition,
CVD (Catalytic Pyrolysis of
hydrocarbons) or Catalytic
Chemical
Vapour Deposition
(CCVD)
4.
Template Carbonization
Method
The
fine details of the
synthetic strategies are
given in the next few
pages.
1.
The Arc-Discharge
Process:
Carbon
nanotubes were first
introduced to the world by
using this synthetic
methodology.
As
stated earlier carbon
nanotubes occurred as a by-product
during the synthesis
of
fullerenes.
Kratschmer and coworkers have
used the same arc discharge
method in 1990
for
the mass production of
fullerene. The method they
have employed comprises of
10.20
Role
of Synthesis in Materials
Technology
evaporating
the graphitic anode. The two
electrodes were made to contact
with each
other
by applying an ac voltage in an inert
gas atmosphere. This results in
the generation
of
an arc that evaporates the
anodic graphite. Thus
fullerenes were generated in
bulk
amounts.
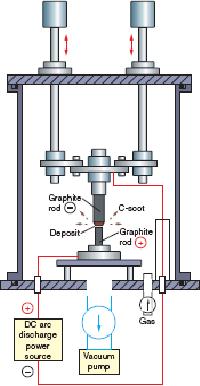
Fig.
10. 10. Schematic
representation of the apparatus
used for the synthesis of
CNT's
[17]
The
arc-discharge apparatus used for
the production of carbon
nanotubes is shown in
Fig.
10.10.
The chamber is first made
free from atmospheric air by
evacuating the
reaction
chamber
with a vacuum pump. Ambient
gas (He or Ar or CH4)
is introduced into
the
chamber.
A dc (direct current) arc voltage is
applied between the two
graphite electrodes
(rods
of carbon). The anodic
graphite evaporates. Fullerenes in the
form of sooth are
deposited
in the chamber. In addition to
fullerenes, the great treasure, CNT's
were also
found
to be deposited on to the cathode from
the evaporating anode. These
CNT's were
found
to be made of coaxial (concentric)
graphene sheets and are termed as
multiwalled
carbon
nanotubes. These results are
obtained when pure graphite
rods free from any
Synthetic
Strategies in Chemistry
10.21
metal
impurities are used as anode
and cathode. By using this arc discharge
method and
by
employing He environment large
scale synthesis of MWCNT's
could be achieved.
If
the synthetic condition is
slightly changed by using metal
catalyst (Fe or Co)
containing
graphite rod as anode instead of
pure graphite rod, single
walled carbon
nanotubes
could be obtained instead of multiwalled
carbon nanotubes. In either
cases the
cathode
is only pure cathode.
Experiments
have been carried out by
changing the
atmosphere
with in the reaction
chamber. Among He, Ar and
CH4,
CH4 gas was found to
be
the best as it resulted in
the formation of highly
crystalline nanotubes with
few
coexisting
nanoparticles which are not
wanted. The major and essential
distinguishing
feature
in the synthesis of fullerens and
CNTs is that, fullerene
cannot be produced
when
reaction
chamber contains hydrogen
containing gases (CH4 or
even H2). At
the same
time
presence of environment of H2 facilitates the formation
of CNT. During the
process
of
arc evaporation in CH4,
thermal decomposition of methane
takes place leading to
the
insitu
generation of H2 as indicated in the
following equation.
C2H2
+ 3 H2
CH4
In
presence of CH4,
the evaporation of graphitic
anode thus takes place in
pure
hydrogen
gas environment. Thus
hydrogen arc discharge is effective in
the generation of
highly
crystalline carbon nanotubes
(multiwalled). As hydrogen arc results in
high
temperature
and resultant high activity,
production of MWCNT's in
hydrogen
atmosphere
(CH4 environment) is more
effective than either He or Ar
atmosphere. Not
only
that, the unwanted carbon
nanoparticles (amorphous carbon)
which are ubiquitous
and
unavoidable can be minimized by using
hydrogen arc process
SWNT's
were synthesized in the year
1993, just two years
after the discovery
of
MWNT's
in the year 1991. SWNT's
were synthesized by the arc discharge
process using
a
graphite anode but with
metal catalyst (either Fe or Co or
metal alloys in some
cases
like
Ni-Y). In sharp contrast to the
synthesis of MWCNT's, SWNT's
were not found on
the
carbon deposit on cathode but were
obtained from the soot in
the gas phase.
Xinluo
Zhao and coworkers has
succeeded in synthesiszing highly
crystalline
SWCNT's
with a clean surface in
large quantities by employing arc
discharge process.
The
SWNT's were obtained with 70
at.% purity.
10.22
Role
of Synthesis in Materials
Technology
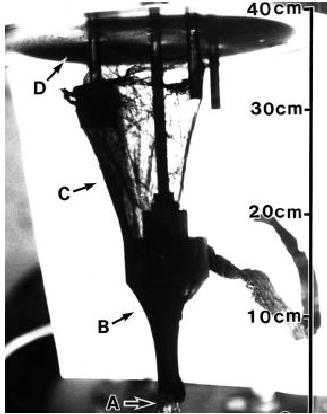
Fig.
10.11. Arc discharge chamber
with a web of SWNT's [18]
(Arrow A shows the
starting
point of the SWNT web;
arrow B shows the black
thick region of
SWNT's;
arrow
C shows the half-transparent
thin region of SWNT's
growth; arrow D
points
towards
the roof of the arc discharge
chamber where the thin
film of SWNT's is
located.
This
film can be peeled of into
large slices)
The
carbon electrode employed comprises of 1
at% Fe catalyst. Inert atmosphere
is
maintained
in the reaction chamber by
H2-Ar mixture. The
catalyst Fe nanoparticles
present
in the product CNT's could
be completely eliminated by heating
the product in
air
at 693 K and also by the subsequent acid treatment
with HCl.
The
two graphite electrodes were
held vertically one over the
other with the anode
at
the
lower end and the cathode at the
upper end separated by 2 mm distance.
The
electrode
assembly was held in the
centre of the vacuum
chamber. The anode is
the
carbon
containing Fe catalyst and the cathode is
pure carbon.
Inert
atmosphere is
maintained
in the reaction chamber by
passing H2-Ar
mixture. The synthesis time
was
roughly
3 mm.
This
has resulted in the
formation of macroscopic web of SWNT's
as
Synthetic
Strategies in Chemistry
10.23
shown
in Fig. 10.11. The length of
the SWNT's is nearly 30 cm.
H2-Ar gas
mixture
played
a crucial role in the
synthesis of SWNT's.
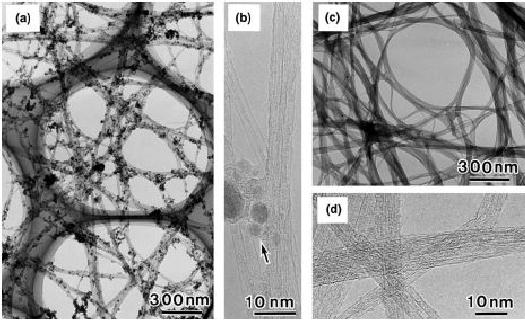
Fig.
10.12. TEM images of (a) as
grown CNT's (low
magnification, iron particles
are
seen
along with tubes), (b) as
grown CNT's (high
magnification, iron particles
are seen
along
with the graphene layers)
(c) purified CNT's (low
magnification, only CTN's
and
no
Fe particles) (d) purified
CNT's (high magnification,
only graphene folded sheets
and
no
Fe particles) [18].
TEM
images of as-grown and purified
SWNT's are shown in Fig.
10.12. Both the
low
magnification and high magnification
images are shown. It is
clear from the TEM
images
that after heat treatment and
the subsequent acid treatment with
HCl the
particulate
impurities (catalyst as well as
amorphous carbon) were
completely removed
yielding
clean and pure
SWNT's.
2.
Laser Furnace Process
The
energy density of lasers is
higher compared to any other
vaporizing device. So
lasers
are
appropriate means to vaporize materials
like carbon with high
boiling temperature
[17].
Typical
laser furnace experimental set up is
shown in Fig. 10.13. The
essential
components
are a furnace, a quartz tube
provided with a window at one end and a
trap for
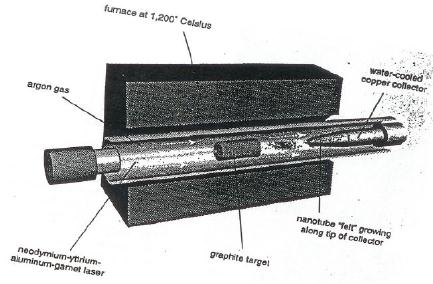
10.24
Role
of Synthesis in Materials
Technology
CNT's
provided with a water
circulation at the other
end, flow system for
inert gas (Ar),
laser
source and the carbon
target.
Fig.
10.13. Laser-furnace (vaporization)
apparatus [19]
The
carbon target used is a
composite of carbon doped
with catalytic Co-Ni alloy.
The
carbon
composite (Co-Ni/graphite) is placed at
the centre of the quartz
tube having
window
at one end through which the laser
beam penetrates into the
quartz tube. The
laser
source used is Nd:YAG (Neodymium :
Yttrium-aluminium-garnet) and this
can
produce
a temperature of 1200 �C in the furnace.
CO2 can also be
used. A laser beam
from
the afore mentioned source is
introduced into the quartz
tube through the
window
and
is focused on to the carbon
composite. Ar gas is circulated
through out the
furnace.
The
carbon composite is vapourized
when the laser beam hit
the target and forms
SWNT's.
The SWNT's produced are
carried to the other end of
the quartz tube
provided
with
a trap (with water
circulation facility) where
the SWNT's are
collected.
It
is important to note that
the surface of the carbon
composite should be kept as
fresh
as
possible for the process of
vaporization to be homogeneous. Since
there is no way the
target
can be moved, the focus of
the laser onto the target
should be changed from time
to
time.
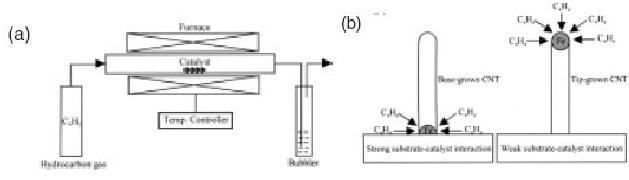
Laser furnace synthesis is an efficient
route to synthesize bundles of
single-walled
carbon
nanotubes with a narrow
diameter distribution.
Synthetic
Strategies in Chemistry
10.25
3.
Chemical Vapour
Deposition
In
this method of synthesis a
hydrocarbon (acetylene, ethylene, benzene
or methane) is
thermally
decomposed in the presence of a
transition metal catalyst
(Fe, Co or Ni) or
catalyst
support (alumina, silica or
zeolite serve as useful supports
for transition
metals)
The
process of synthesis can be carried
out even at relatively lower
temperatures (600-
1200
�C) than those which are
normally encountered in either arc
discharge process or
laser
vaporization processes.
But
we have to forgo the quality
of the nanotubes
obtained
interms of crystallinity when
the synthesis temperatures are
lower. Since this
synthetic
methodology can be employed at relatively
lower temperatures and
ambient
pressure,
CVD is simple and also forms a
viable means of producing
large amounts of
nanotubes.
Hydrocarbons
in either liquid (benzene,
alcohols) or solid
(camphor,
napthelene)
or gaseous (CH4 or C2H2) can be virtually
employed as carbon
precursors.
Fig.
10.14. Experimental set up
for Chemical Vapour
Deposition Synthesis, (b)
probable
models
of CNT growth [17]
The
experimental set up used for
a CVD or CCVD (catalytic
chemical vapour
deposition)
synthesis
is shown in Fig. 10.14 (a)
and the possible ways in which
the nanostructures
grow
on catalyst particles is represented
pictorially in Fig. 10.14
(b).
In
a typical process of CVD,
the catalyst is placed in the
middle of a quartz
tube.
The
quartz tube with the
catalyst is placed in a furnace capable
of generating and
sustaining
temperatures between (600-1200 �C).
The hydrocarbon vapour is
allowed to
pass
through the quartz tube
containing catalyst material present at
sufficiently high
10.26
Role
of Synthesis in Materials
Technology
temperature.
The hydrocarbon gets
decomposed. CNT's start
growing on the
catalyst
particles.
The temperature of the
furnace is then cooled to
room temperature. The
CNT's
are collected. If the carbon
precursors were to be in liquid form as
in the case of
either
benzene or alcohols then an inert
gas like Ar is bubbled
through the flask
containing
the liquid
hydrocarbon.
The
liquid hydrocarbon in the
flask is heated
simultaneously
so that vapours of the
hydrocarbon are generated. The
vapours of the
hydrocarbon
are thus carried by the
inert gas through the
catalyst particles located in
the
hot
zone of the furnace. If the
carbon precursors were to be in
solid state like those
of
naphthalene
or camphor, the hydrocarbon
vapours in such cases are
generated by placing
them
in another furnace kept at a
lower temperature prior to
the main furnace where
the
deposition
of carbon takes place over
the catalyst particles.
Analogous to the
carbon
precursors
the catalyst materials can also be in
either solid or liquid or
gaseous state. In
the
high temperature zone the
hydrocarbon vapour gets
decomposed. A variety of
carbon
species
are formed.
Such
carbon species are capable
of dissolving in the
metal
nanoparticles
of the catalyst. Once a
catalyst particle is supersaturated,
carbon species
starts
precipitating out. Initially,
fullerene dome like structure will
petrude out of the
catalyst
particle which extends into
carbon cylinders. The
position and direction of
growth
of carbon from the metal
nanoparticle depends on the
interaction between
the
metal
particle and the support .If
the interaction beween the
metal particle and the
support
is
strong, the particle is
literally immovable. The
decomposed carbon species will
be
adsorbed
into the particle initially
from all the direction
where the catalyst
particle
surface
is exposed to the carbon
species. Once a level of super
saturation of the
particle
with
carbon species is reached no
more carbon species are
adsorbed in to the
metal
particle.
Now carbon has to
precipitate out from the
metal particle. Since the
particle is
strongly
held by the support, the
only direction in which the
carbon can precipitate out
is
from
the tip. The process of
dissolution now takes place
from the sides of the
particle
near
the base and the excess
carbon is precipitated at the
mouth or tip of the
particle.
Since
the grown process starts at
the sides of the base
this is known as base
grown
carbon.
On the contrary, if there
were to exist weak
interaction between the
catalyst
particle
and the support, the
particle can now be easily
lifted vertically up above
the
support
surface the movement when
the particle gets supersaturated
with carbon that is
Synthetic
Strategies in Chemistry
10.27
being
absorbed from the tip. Since
the growth of the tube is
now originating at the tip
of
the
metal particle the mechanism
is regarded at tip grown CNT
process. Depending on
the
size of catalyst particle, we get
either SWNT's or
MWNT's.
4.
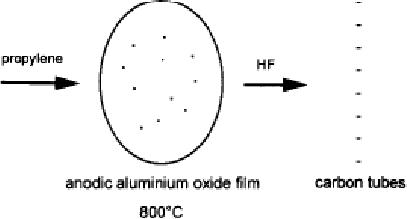
Template Carbonization
Method:
The
structure directing material
(template) used for
preparing nanotubes in this
method is
the
porous aluminium plate.
Channels are created in the
aluminium plate by the
process
of
anodic oxidation in the
presence of sulphuric acid.
Carbon is deposited
pyrolytically
onto
the channels in aluminium
oxide film at 800 �C in inert
atmosphere.
Fig.
10.15. Synthesis of carbon
nanotubes by template carbonization
method [12]
After
the process of carbon
deposition and subsequent carbonization
the aluminium oxide
template
is removed by treatment with HF
(hydrofluoric acid) solution.
(Note: HF should
be
handled with utmost care.
Proper hand gloves should be
used while handling HF. It
is
so
hazardous that through skin
it can damage even the bones).
This resulted in
uniform
monodisperse
carbon nanotubes with
uniform length, diameter and
thickness. As the
temperature
of the carbonization is not
high (800 �C only), the
tubes contained structural
imperfections.
Typical synthetic steps
involved in a template assisted
synthesis are
pictorially
represented in Fig.
10.15.
10.28
Role
of Synthesis in Materials
Technology
Can
a carbon source as common as
kerosene be used for the
synthesis of nanoforms
of
carbon materials?
Kerosene
is used as a fuel for
cooking and lightening. It is a residue of
the petroleum
refinery.
Kerosene contains a mixture of various
short and long chain
aromatic and
aliphatic
hydrocarbons.
Sharon
and coworkers have used
kerosene as precursor
for
preparing
various nanoforms of carbon.
The method of synthesis comprises of
pyrolysis
of
kerosene at 1000 �C. The
experimental arrangement comprises of a
quartz tube held
horizontally
in a furnace.
Kerosene
is placed in a round bottom flask and
heated
thermostatically
at 90 �C. The vapours of
kerosene along with Ar gas
were allowed to
pass
through the quartz tube
held in a furnace. Stainless steal plates in
rectangular shape
were
placed in the center of the
quartz tube. The stainless steal
plate serves as a substrate
for
the deposition of various
forms of carbon materials.
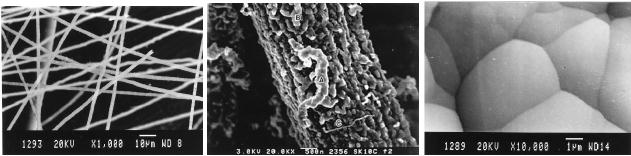
Straight, stiff and long
fibers,
flexible,
thin hair like threads, soft
wool like clusters of
carbon, uniform
nanotubes,
bitter-gourd-like
rough fibers, earth
worm-like nanofibers and carbon
thin film deposits
were
found around and on the stainless steal
substrate.
SEM
(scanning electron
microscope)
images of the various
nanoforms of carbon synthesized
are shown in Fig.
10.16.
It is to be noted that the substrate
stainless steal is a complex catalyst of
different
compositions
which govern the growth and
orientation of the nanostructures.
Important
questions
one should now consider are:
How a single carbon
precursor under given
conditions
of synthesis yields different
nanostructures simultaneously? What
factors are
responsible
for control of size and shape of
the nanostructures? Can each of
the different
carbon
nano structures be separated?
How does the synthetic
strategy adopted dictates
the
ultimate materials technology? Or in
other words, what impact
does this synthetic
strategy
have on the ultimate
materials technology? The
ultimate materials technology
is
a
function of material structure as
well as material property
which are controlled by
the
specific
synthetic strategy
employed.
For
instance, as in the present case,
the fibers
obtained
were found to be conducting and
such conducting fibers are
useful in
biosensors,
ion-activated molecular switches and
for the fabrication of
microelectrodes
for
medicinal applications. Composite
fibers can be obtained from
hair-like and wool-
like
fibers. The bitter gourd
like fibers with extremely
irregular outer surface
yields large
specific
surface area values and will be
useful for catalytic
applications. The electrodes
Synthetic
Strategies in Chemistry
10.29
fabricated
from the thin film
carbon synthesized was found to be a
substitutes to an
expensive
RuO2 coated
titanium sheet which is used
as anode against a mercuric
cathode
for
the electrolysis of brine
solution. The electrochemical
potential range of the
electrode
fabricated
from carbon thin film
material was found to be -1.24 to
1.67 V Vs SCE
(standard
calomel electrode) indicating
that water will not be
electrolysed by using
the
electrodes
(both anode and cathode)
fabricated by this carbon
material. The electrodes
fabricated
from thin film carbon
could electrolyze 30 % NaCl
solution at 300 mAcm-2 for
more
than 110 h continuously with
out any deterioration [21].
Thus the electrodes
fabricated
from the carbon obtained by
kerosene pyrolysis are
useful for
Chloro-alkali
industry
offering a solution for
toxicity problem by eliminating
the hazardous mercury
contamination
[20].
(a)
(b)
(c)
Fig.
10.16. SEM (scanning
electron microscope) images of
(a) hair like fibers,
(b) bitter
gourd - like rough fibers
and (c) carbon thin film
grown on a stainless steel substrate
Carbon
Nanotubes as STM and AFM
Tips:
Gerd
Binnig and Heinrich Rohrer at
the IBM (International Business
Machines
Corporation)
research laboratories in Zurich in
the late 1970's developed a
method of
structural
analysis namely the STM,
scanning tunneling microscopy.
Few details on the
principle
of operation of STM are
worth knowing. Electron is
not only a particle but
also
a
wave. In our daily life
walking through a brick wall
is not possible [7]. Imagine
a
nanometer
scale equivalent of a brick
wall. Let this be an energy
barrier that an
electron
following
prequantum physics would not
be able to overcome. But quantum
mechanics
tells
us that there is still a
certain probability that the
electron is found on the
other side of
the
wall since electron is not
only a particle but also a
wave. In this case the
electron
10.30
Role
of Synthesis in Materials
Technology
behaves
more like a wave and this
effect is called tunneling
which forms the basis
of
STM
analysis. The empty space
between the surface to be
studied and the probe is
regarded
as the barrier or the wall
through which an electron
normally but
occasionally
will
not passes. The tip of
the probe should be one atom
wide. The next layer
can
contain
more atoms. The probability
of tunneling decreases by a factor of
ten for each
0.1
nm of additional distance. So the second
layer has no virtual
significance. The one
atom
tip is suspended above the
object just a few atomic
radii. This distance is
readjusted
using
the measured tunneling
current. From the distance
between the STM tip and
the
surface
to be probed and the measured tunneling
current one can feel the
structure of the
surface.
The peaks and troughs of the
surface can be felt at atomic
scales with out
ever
touching
it. It has now become
possible to control the placement of
the tip with
sufficient
accuracy
establishing STM as a standard method of
analysis in materials science.
A
variation of the theme of
electron tunneling found as in
the case of STM was
presented
in 1986 by Binnig, C. F. Quate and C.
Gerber which was latter
called as
Atomic
Force Microscopy (AFM). In this
technique the probe will actually
touch the
surface.
The vertical movement of the
tip of the probe will be controlled by
the repulsive
force
measured when the surface is
touched. By 1990's individual
biomolecules such as
the
double-stranded DNA could be pictured
which made the researchers
elated. Thus
both
STM and AFM techniques are in
principle suitable for
"feeling" the fine structure
of
a
surface atom by atom.
The
performance of STM and AFM instruments
has been limited by the
quality of the
tip
which acts as a probe that
either exchanges electron with
the surface as in the case
of
STM
or touches the surface as in
the case of AFM. The
virtual characteristics a
tip
should
possess to be used as probe are: such
tips should end in a single
atom; possess a
well
defined geometry, should be
conductive and chemically inert. So
far the tool
that
worked
as a good probe tip is obtained by simply
cutting a metal wire with an
ordinary
pair
of scissors.
Is
it not surprising?
Even
though the afore mentioned
material
possessed
no such virtual attributes as
mentioned previously for an
ideal probe, the
material
derived by cutting through a
metal wire with a pair of
scissors worked very
well.
The
diameter of such a tip is of a
few hundred nanometers indicating
that the outer
most
Synthetic
Strategies in Chemistry
10.31
layer
contains thousands of atoms.
Interestingly, alternative methods
like etching the
wire
tips or some other
improvements in shaping the
metal wire only failed to
function.
Tremendous
improvements have been
brought about in the
advancement of utility of
STM
and AFM techniques for probing
the surface structure when
carbon nanotubes
(CNT's)
were used for the
first time as AFM tips by
the researchers from the
laboratory
of
Nobel Laureate Richard
Smalley. A single carbon
nanotube of 10 nm wide and 100
nm
to 1 �m long capped with
fullerene like hemisphere is glued
as molecular antenna
onto
a conventional probe. The
conventional probe was coated initially
with a suitable
glue
and then dipped into a bundle of
nanotubes. In most cases,
this effort
culminated
into
the gluing of just one tube
onto the tip.
The
new probe onto which carbon
nanotube is glued fulfilled
the ideal conditions
the
STM
or AFM tip should satisfy.
The probe with CNT tips
has a well-defined and
well-
known
molecular structure, is conductive,
chemically inert and also very
thin. Use of
carbon
nanotubes as AFM tips provided
additional advantages by being stable
to
withstand
the forces applied to the
tip and also being elastic
enough to avoid
unwanted
collisions
with the surface. More
over, now-a-days, reliable and
reproducible synthetic
strategies
are at disposal for making
such new probes (carbon
nanotubes).
Role
of Synthesis in Leather
Technology:
Transformation
of animal hides and skins into
attractive, aesthetic and useful
artifacts has
been
one of the oldest technologies of
mankind.
When
wet the animal skins
are
susceptible
to bacterial attack and
putrefy.
On
the contrary drying makes
the skin
inflexible
and useless for clothing and
other applications. Such
problems can be over
come
by the use of bactericide
during soaking . This forms
a good solution for the
short
term
preservation of skins and hides. Addition
of bactericide prevent the bacterial
attack
on
hide and skin. Addition of bactericide
convert the putrescible
biological materials
into
a
stable material resistant to microbial
activities with enhanced resistance to
wet and dry
heat.
The bactericide kill the
growth of microorganism there by
preventing the damage
to
skin and hide.
Mercury
compounds as well as a mixture of
sulfite and acetic acid were
extensively
used
as bactericides. Even though the
afore mentioned materials
are effective they
are
damageable
and harmful to the environment. So
they are no longer used
for protecting
10.32
Role
of Synthesis in Materials
Technology
the
hides and skins from bacterial
attack. Instead, bronopol
which is a highly
active
antimicrobial
chemical compound has been
the ubiquitous choice to
leather industry to
prevent
bacteria attacking the skins and
hides. Bronopol is 2-bromo-2-nitro
propane 1,
3
diol. Bronopol was invented by
The Boots Company PLC,
Nottingham, England in
the
early 1960's. It was used as a
preservative for pharmaceuticals
for the first time
[21].
Earlier
reports on the synthetic
path ways of bronopol
include reaction of
bromo-nitro-
methyl-cyclohexanol
with aliphatic aldehyde. The
main drawbacks associated
with this
method
of synthesis are the use of
cyclohexanol which is expensive and also
the use of
hazardous
sodium ethoxide. Lakshimi
Muthusubramanian and Rajat B Mitra
[22] have
succeeded
in deloping a simple but
cleaner synthetic strategy
that helped Leather
manufacturing
technology.
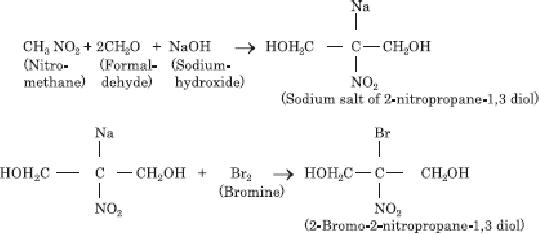
Scheme
10.1. Synthetic strategy for
Bronopol
This
method of synthesis of bronopol
includes reaction of formaldehyde
with nitro alkane
in
the presence of sodium
hydroxide (in MeOH) which on
bromination yielded
bronopol
as
represented in Scheme
10.1.
The
advantages of this method
include complete
replacement
of hazardous and expensive hydroxide and
methanol which are
inexpensive
and
readily available. Currently
scale up studies are being
carried out for the
production
of
bronopol using this
environmentally benign synthetic
strategy.
CONCLUSION:
The
living cells are versatile
in its design and function. They
provide all necessary
inspiration
to design and synthesize new materials
with specific functions that
bring
Synthetic
Strategies in Chemistry
10.33
about
revolutions in Technology and also give
birth to Advanced
Technologies.
Understanding
and imitation of natural machinery of
the living cells holds great
rewards.
But
such endeavours are not free
from barriers and obstacles. For
instance, molecular
level
details of the working of ribosomes
(their function of protein
synthesis) is unclear
even
today and remains one of the hardest
problems in biology.
Therefore
our
knowledge
and understanding of the processes
going on in a living cell and also
the
mechanochemical
functions of various cell
components is limited. Any improvements
in
such
an understanding facilitate imitation and
mimicking of the synthetic
strategies
involved
in life process (a unique
network of chemical reactions).
This knowledge will
in
turn bring about drastic
changes and advancements in Materials
Technology.
REFERENCES:
1.
http://en.wikipedia.org/wiki/cell_(biology)
2.
http://www.ucmp.berkeley.edu/history/hooke.html
3.
http://www.roberthooke.org.uk/
4.http://images.google.co.in/imgres?imgurl=http://cache.eb.com/eb/image%3Fid%3D997
68%26rendTypeId%3D4&imgrefurl=http://www.britannica.com/ebc/art-99713/Robert-
Hookes-drawings-of-the-cellular-structure-of-cork
and&h=450&w=339&sz=44&hl=en&start=1&um=1&tbnid=8NZeLWw7jw9MzM:&tbn
h=127&tbnw=96&prev=/images%3Fq%3DRobert%2BHooke%2Bcell%26um%3D1%26
hl%3Den%26sa%3DN
5.
http://www.cellsalive.com/cells/3dcell.htm
6.
Travels to the nanoworld
Miniature Machinery in Nature and
Technology, Michael
Gross,
Perseus Publishing, Cambridge,
Massachusetts, 1999.
7.
North Central Regional
Eductional Laboratory, 1-800-356-2735,
1997-99.
8.
http://www.sp.se/en/areas/material/Sidor/default.aspx
9.
http://en.wikipedia.org/wiki/Gutenberg_Bible
10.
Warren H. Hunt, Jr. JOM,
October 2004, 13.
11.
H. W. Kroto, J. R. Heath, S. C. O'Brien,
R. F. Curl and R. E. Smalley, Nature
318
(1985)
162
12.
P. Scharff, Carbon 36 (1998)
481
13.
T. Pradeep, Current Science, 72
(1997) 124
10.34
Role
of Synthesis in Materials
Technology
14.
Marc Monthionx, Vladimir L.
Kuznetsov, Carbon 44 (2006)
1621
15.
Purushottam Chakraborty, Everyman's
Science, Vol. XXXIX, No. 4,
October-
November'
04
16.
Nanotube and Nanofibers, Yury Gogotsi
(Ed.,) Taylor & Francis,
2006
17.
Yoshinori Ando, Xinluo Zhao,
Toshiki Sugai, and Mukul
Kumar, Materials
Today,
October
2004, 22-29
18.
Xinluo Zhao, Sakae Inone,
Makoto Jinno, Tomoko Suzuki,
Yoshinori Ando,
Chemical
Physics Letters, 373 (2003)
266
19.
R. Saito, G. Dresselhaus and M. S. Dresselhaus,
Physical Properties of Carbon
Nanotubes,
Imperial College Press,
1998
20.
Mukul Kumar, P. D. Kichambare,
Maheshwar Sharon, Yoshinori
Ando and Xinluo
Zhao,
Materials Research Bulletin 34
(1999) 791
21.
http://en.wikipedia.org/wiki/Bronopol
22.
Lakshmi Muthusubramanian, Rajat B
Mitra, Journal of cleaner
production, 14 (2006)
536
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES