 |
INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS |
| << PREFACE:Content |
| SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE >> |
Chapter
- 1
INTRODUCTION
TO SYNTHETIC STRATEGIES IN
CHEMISTRY
Prof. B.
Viswanathan
INTRODUCTION
One of
things that make chemistry
unique among the sciences is
the synthesis.
Chemists
make
things, new pharmaceuticals,
food additives, materials,
agricultural chemicals,
coatings,
adhesives, and all sorts of useful new
molecules. The chemists
prepare them
from
simpler more readily
available starting materials.
There are two aspects to
organic
synthesis
first the development of a
synthetic strategy or plan of
action and the second
the
actual
implementation of that plan in a
chemical laboratory.
Synthesis
of molecules and materials are
important aspect of the
development of
science.
In the past, synthesis is
based on some of the Name
reactions in organic and
Inorganic
chemistry.
The
attempts to synthesis new
molecules and materials
have
always
depended on the experience and
extrapolation of the existing
knowledge to new
situations
and new synthesis of
molecules.
However
in the last two decades,
the
synthesis
has become a well established
science going beyond the
recollection and
adoption
of the existing procedures. The
normal procedures so far adopted
for the
synthesis
of materials are
(i)
precipitation
based on solubility principle (
based on thermodynamic
quantities)
(ii)
Bond
formation and bond breaking
usually brought about by
reagents,
participating
species and leaving entities (these
are often kinetically
controlled);
processes depending on the
strength of binding already
existing
or
being formed.
(iii)
simple
solid state reactions often
controlled by diffusion
(iv)
ligating
or binding species
These
procedures have been satisfying
the normal curiosity of the
chemists for new
molecules.
However
today, Chemistry is the
study of molecules, materials
of
functionalities
and hence they have to be synthesized,
built, and architectured and
designed
1.2
Introduction
to Synthetic Strategies in
Chemistry
Today
the molecules and materials
are for many applications..
Every sector of life
requires
molecules/materials with functionalities,
stability, as well as durability
under
adverse
conditions. Molecules of highly
active functionalities with
stability, durability
and
appropriate stress- strain relationship
(global properties of dissolution,
digestion,
attachment
to species and others) are
the demands of today.
Most
often the materials
required
have to be in the meta stable state
but should be stable enough to be
useful in
devices.
The
exploitable properties must be able to be
controlled by Size,
shape,
orientation
and morphology.
Over
the last thirty years,
synthetic chemists
have
developed
an assortment of routes to obtain
optically pure compounds.
Many strategies
include
the use of pure starting
materials with chiral centres. In
addition, the utilization
of
chiral
auxiliary groups have been
implemented to achieve an increase in
stereo-selectivity
and
to simplify the purification
process. Lastly, asymmetric
catalysis using enzymes
or
inorganic
catalysts has also been used
to afford the desired
stereochemistry.
At
this stage the important
questions to be faced are:
1.
In the case of organic
chemistry how can one build
the desired carbon
skeleton?
2.
How does one introduce the
necessary functional groups?
3.
How does one control the
regio- and stereochemistry of
reactions?
These
questions can be expanded to any extent
depending on the need for
molecules
and
materials.
The
chemical synthesis of complex
organic molecules is integral to
many advances
that
enhance the quality of life,
such as novel disease
treatments, agrochemicals
with
improved
properties and advanced materials for
high performance technology
and
biotechnology.
The
methods for synthesizing
complex organic molecules
have
traditionally
pieced molecules together in a
linear manner, gradually
building complexity
into
the molecule. This can be a
very time consuming process,
and synthetic routes to
molecules
can end up being incredibly long,
requiring extensive resources
and
manpower.
Since
2000 pioneering approaches to synthesis
that combines
two-directional
synthesis
and tandem reactions. Linear
symmetrical trifunctional compounds
are
synthesized
through use of two-directional
synthesis and a range of tandem reactions
are
then
applied to generate a range of diverse
structures from these simple
substrates. Two-
Synthetic
Strategies in Chemistry
1.3
directional
synthesis, when used in
combination with tandem
reactions, can lead to
significantly
faster strategies for the
synthesis of complex
molecules.
The
recognition of the strong
dimensionality-dependent physical-chemical
properties
of
inorganic matter at the nanoscale
has stimulated efforts
toward the fabrication
of
nanostructured
materials in a systematic and controlled
manner. Surfactant-assisted
chemical
approaches have now advanced to
the point of allowing facile
access to a
variety
of finely size- and shape-tailored
semiconductor, oxide and metal
nanocrystals
(NCs)
by balancing thermodynamic parameters and
kinetically-limited growth
processes
in
liquid media. While refinement of
this synthetic ability is
far from being
exhausted,
further
efforts are currently made
to provide nanocrystals with
higher structural
complexity
as means to increase their
functionality. By controlling crystal
miscibility,
interfacial
strain, and facet-selective reactivity at
the nanoscale, hybrid nanocrystals
are
currently
being engineered, which consist of
two or more chemically
different domains
assembled
together in a single particle
through a permanent inorganic
junctions.
POROUS
MATERIALS
This
is one class of materials which
has seen a tremendous
advancement in the
synthetic
strategies.
Porous solids have high
scientific and technological interest.
They are able to
interact
with atoms, ions and
molecules at surfaces and throughout the
bulk of material.
Distribution
of sizes, shapes and volumes of
the void spaces in porous
materials are
directly
related to their ability to
perform desired function in
particular application.
High
SA/volume ratio provides a
strong driving force to
speed up thermodynamic
processes
that minimize free
energy
In
high surface area materials
the active sites are
more isolated. Materials
with uniform
pores
can separate molecules on basis of
size. They are also employed
as
adsorbents,
catalyst
supports, and electrode
materials.
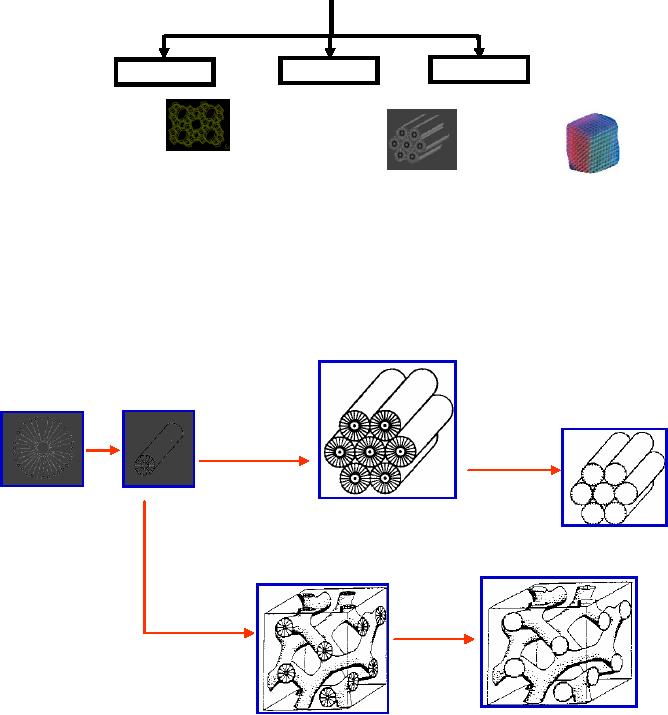
The
porous solids can be classified
according to the range of pore sizes
available in them.
A
simple classification is shown in
Scheme 1.1.
1.4
Introduction
to Synthetic Strategies in
Chemistry
Porous
materials
macroporous
mesoporous
Microporous
eg:
ZSM-5,AlPO
MCM-48,SBA-15,CMK-n
photonic
crystals
P.D
<
2 nm
2-50
nm
>
50 nm
300-500
m2g-1
1000-3000
m2g-1
10-500
m2g-1
SA:
Scheme
1.1 A simple classification of
porous substances.
The
porous architecture is normally
built in various ways. One
such methodology is
shown
in Fig.1.
Schematic
representation
Silica/surfactant
=
1/0.27
calcination
As-synthesized
MCM-41
(hexagonal
structure)
MCM-41
Silica/surfactant
=
1/0.60
calcination
As-synthesized
MCM-48
MCM-48
(cubic
structure)
J.
S. Beck, J.C. Vartuli, W. J.
Roth, M. E. Leonowiez, C. T. kresge, K.
D. Schmitt, C. Chu, D. H. Oison,
and E. W.
Sheppard.
S. B. McCullen, J.L. Schlenker. J.
Am. Chem. Soc.,
114 (1992) 10834
Fig.
1.1. The formation of mesoporous
Mobil composition of materials
(MCM) formed as
a
result of template mechanism
(the details will be discussed in
chapter 6)
Synthetic
Strategies in Chemistry
1.5
Conventionally
the template route is
understood in terms of space
filling mechanism as
shown
in Fig. 2.2 by the template
molecules, template molecular
aggregates or structure
directing
agents.
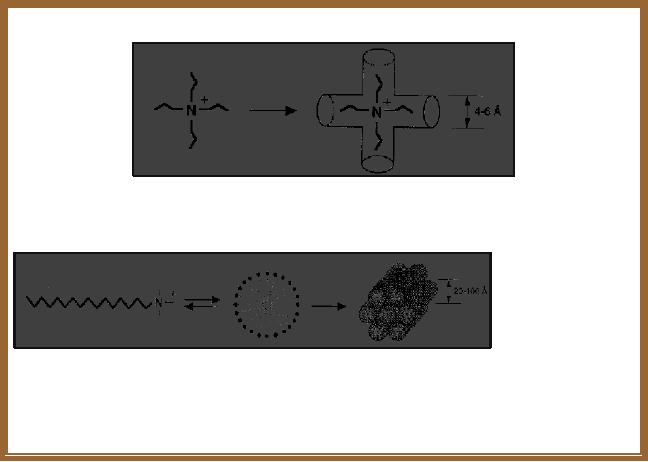
The
role of quaternary directing
agents
Small
individual alkyl chain
length quaternary directing agents
generate the formation
of
micro-porous solids
Long
alkyl chain length
quaternary directing agents
self-assemble to supramolecular
Species
which can generate the
formation of mesoporous molecular
sieves
Thomos
J. Barton et al., Chem
Mater., 11 (10) ( 1999)
2633
Fig.
1.2. Simple mechanism for
the formation of porous
solids
Generally
the interactions in formation of
materials can be understood in terms
of
bonding
interactions. One such
classification is given in Scheme
1.2.
ORGANIC
SYNTHESIS
Classic
examples of synthesis can be found in
the area of organic
Chemistry. Strategies
and
Tactics in Organic Synthesis
provide a forum for
investigators to discuss
their
approach
to the science and art of
organic synthesis. Rather
than a simple presentation
of
data
or a second-hand analysis, one is
provided with stories that
vividly demonstrate
the
power
of the human endeavour known
as organic synthesis and the
creativity and tenacity
of
its practitioners. First
hand accounts of each
project tell us of the
excitement of
1.6
Introduction
to Synthetic Strategies in
Chemistry
conception,
the frustration of failure and
the joy experienced when either
rational thought
and/or
good fortune give rise to successful
completion of a project. Synthesis is
really
done
and are educated, challenged and inspired
by these stories, which portray
the idea
that
triumphs do not come without
challenges. We also learn that we can
meet challenges
to
further advance the science and
art of organic synthesis,
driving it forward to meet
the
demands
of society, in discovering new
reactions, creating new
designs and building
molecules
with atom and step economies
that provide solutions
through function to
create
a
better world.
These
are usually termed as Name
reactions in organic chemistry.
These
aspects
will be dealt with in separate
chapters.
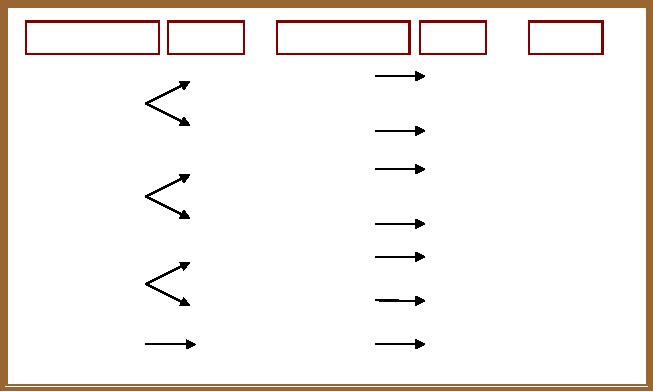
Type
of interaction
Surfactant
Inorganic
precursor
Notation
Examples
S+-----I-
Cationic
+
Anionic
M41S,
Ionic
M-MCM-41,
48
(Direct
pathways)
Cationic
Anionic
+
S------I+
M-M41S,
S+ X-I+
Cationic
+
Cationic
SBA,
APM
Ionic
(Mediated
pathways
)
Metal
Oxides
S- M+ I-
Anionic
Anionic
+
HMS
S0-----I0
Neutral
+
Neutral
Hydrogen
Amine
bonding
(Neutral
)
SBA
S0-----I0
Neutral
+
Neutral
Polymer
Covalent
TMS
Neutral
+
Neutral
S-----I
Scheme
1.2. Possible interactions in
the Synthetic Strategies for
Porous Materials
META
STABLE STATE OF THE MATERIALS
For
many device and other
applications, one is required to make
materials in the meta
stable
state.
Meta
stable state can be obtained in materials
by a variety of routes.
The
possible
list of the methods can
be:
�
Organization
�
Templating
bonding and structural
�
Auto-thermal
Synthetic
Strategies in Chemistry
1.7
�
Transport
CVD, PVD
�
Field
induced ( electro-deposition, T
induced)
�
Moulding
and blowing
�
Extrusion
( geometry control)
The
list given is not complete
but it gives an idea of what
methods can generate the
meta
stable
of materials.
INORGANIC
MATERIALS
Inorganic
materials occupy a unique place and
they can be synthesized by
various
methods.
Some well known methods
include
Sol
gel techniques
Co-ordination
chemistry
Weak
interactions
bonding
interactions
These
methods are described in
detail in subsequent chapters. The purpose of
the present
book
is to assimilate available information on
synthetic strategies in Chemistry.
Though
one
can not claim comprehensiveness on this
topic, it is the endeavour to
bring some of
the
available knowledge in one place. It is hoped
from that point of view,
the synthetic
chemists
may find this book
useful.
Table of Contents:
- INTRODUCTION TO SYNTHETIC STRATEGIES IN CHEMISTRY:POROUS MATERIALS
- SYNTHETIC METHODS BASED ON ACTIVATING THE REACTANT:HALOGENATION OF BENZENE
- METHODS BASED ON ACTIVATING THE REACTING SUBSTANCE:Experimental method
- SYNTHESIS OF MATERIALS BASED ON SOLUBILITY PRINCIPLE
- SOL-GEL TECHNIQUES:DEFINITIONS, GENERAL MECHANISM, INORGANIC ROUTE
- TEMPLATE BASED SYNTHESISSynthesis, Mechanism and Pathway
- MICROEMULSION TECHNIQUES:Significance of Packing Parameter
- SYNTHESIS BY SOLID STATE DECOMPOSITION:DECOMPOSITION METHODS
- NEWER SYNTHETIC STRATERGIES FOR NANOMATERIALS:Nanostructured Materials
- THE ROLE OF SYNTHESIS IN MATERIALS TECHNOLOGY:The Holy Bible
- ELECTROCHEMICAL SYNTHESIS:FEATURES OF ELECTROCHEMICAL SYNTHESIS
- NEWER REACTIONS AND PROCEDURES: CATALYTIC AND NONCATALYTIC
- SYNTHETIC STRATEGIES - FROM LABORATORY TO INDUSTRY
- SYNTHESIS OF CHEMICALS FROM CARBON DIOXIDE:Carbon dioxide - Dry Ice
- CARBOHYDRATES TO CHEMICALS:MONOSACCHARIDES
- SOME CONCEPTUAL DEVELOPMENTS IN SYNTHESIS IN CHEMISTRY
- COMPUTATIONAL BASICS UNDERLYING SYNTHETIC STRATEGIES