160 micrograms/4.5 micrograms/inhalation, inhalation powder
budesonide/formoterol fumarate dihydrate
What Symbicort Turbuhaler is and what it is used for
Symbicort Turbuhaler is an inhaler used to treat asthma in adults and adolescents aged 12–17 years. It is also used to treat symptoms of chronic obstructive pulmonary disease (COPD) in adults aged 18 and over. It contains two different medicines: budesonide and formoterol fumarate dihydrate.
- Budesonide belongs to a group of medicines called “corticosteroids”. It works by reducing and preventing swelling and inflammation in the lungs.
- Formoterol fumarate dihydrate belongs to a group of medicines called “long-acting beta 2 – adrenoreceptor agonists” or “bronchodilators”. It works by making the muscles in your airways relax, helping you breathe more easily.
Asthma
Symbicort Turbuhaler can be prescribed for asthma in two different ways.
a) Some people have been prescribed two asthma inhalers: Symbicort Turbuhaler and a separate attack suppressor.
- They use Symbicort Turbuhaler every day, which helps prevent the onset of asthma symptoms.
- They use their inhaler when they have asthma symptoms to make it easier to breathe again.
b) Some people are prescribed Symbicort Turbuhaler as their only asthma inhaler.
- They use Symbicort Turbuhaler every day, which helps prevent the onset of asthma symptoms.
- They also use Symbicort Turbuhaler when they need an extra dose to treat asthma symptoms and make it easier to breathe again and, if agreed with the doctor, also to prevent the onset of asthma symptoms (for example when they exercise or are exposed to allergens). They do not need a separate inhaler for this.
Chronic Obstructive Pulmonary Disease (COPD)
Symbicort Turbuhaler can also be used to treat the symptoms of COPD in adults. COPD is a long-term disease of the airways that has often been caused by cigarette smoking.
What you need to know before you use Symbicort Turbuhaler
Do not use Symbicort Turbuhaler
- if you are allergic to budesonide, formoterol, or any of the other ingredients of this medicine (listed in section 6), in this case, lactose (which contains small amounts of milk protein).
Warnings and precautions
Talk to your doctor or pharmacist before using Symbicort Turbuhaler if:
- You have diabetes.
- You have a lung infection.
- You have high blood pressure or if you have ever had heart problems (including irregular heartbeat, very fast pulse, narrowing of the arteries, or heart failure ).
- You have problems with the thyroid gland or with the adrenal glands.
- You have low levels of potassium in your blood.
- You have severe liver problems.
Contact a doctor if you experience blurred vision or other visual disturbances.
Other medicines and Symbicort Turbuhaler
Tell your doctor or pharmacist if you are using, have recently used, or might be using other medicines.
In particular, you should tell your doctor or pharmacist if you are using any of the following medicines:
- Beta-blockers (such as atenolol or propranolol for high blood pressure ), including eye drops (such as timolol for glaucoma ).
- Medicines for fast or irregular heartbeat (such as quinidine ).
- Medicines such as digoxin are often used for heart failure.
- Diuretic drugs (such as furosemide ). These are used against high blood pressure.
- Steroids are taken by mouth (such as prednisolone ).
- Xanthines (such as theophylline or aminophylline). These are often used to treat asthma.
- Other bronchodilators (such as salbutamol ).
- Tricyclic antidepressants (such as amitriptyline) and the antidepressant nefazodone.
- Phenthiazines (such as chlorpromazine and prochlorperazine).
- Medicines called “HIV protease inhibitors” (such as ritonavir) for HIV infection.
- Anti-infection medicines (such as ketoconazole, itraconazole, voriconazole, posaconazole, clarithromycin, and telithromycin).
- Medicines for Parkinson’s disease (such as levodopa ).
- Medicines for thyroid problems (such as levothyroxine).
If any of the above applies to you, or if you feel unsure, you should talk to your doctor or pharmacist before you start using Symbicort Turbuhaler.
If you will need anesthesia in connection with an operation or a dental procedure, you must tell your doctor or pharmacist.
Pregnancy, breastfeeding, and fertility
- If you are pregnant or planning to become pregnant, consult a doctor before using Symbicort Turbuhaler – do not use Symbicort Turbuhaler unless your doctor advises you to do so.
- If you become pregnant while using Symbicort Turbuhaler, do not stop using Symbicort Turbuhaler but contact your doctor immediately.
- If you are breastfeeding, consult your doctor before using Symbicort Turbuhaler.
Driving ability and use of machinery
Symbicort Turbuhaler has no or negligible effect on your ability to drive or use tools or machines.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased vigilance. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and side effects. Description of these effects and side effects can be found in other sections of the package leaflet. Read all the information in this leaflet for guidance. Discuss with a doctor or pharmacist if you are unsure.
Symbicort Turbuhaler contains lactose
Symbicort Turbuhaler contains lactose which is a type of sugar. If you have an intolerance to some sugars, you should consult your doctor before using this medicine. The amount of lactose in this medicine does not normally cause problems in people who are lactose intolerant.
The excipient lactose contains small amounts of milk protein, which can cause allergic reactions.
How to use Symbicort Turbuhaler
- Always use this medicine as directed by your doctor. Ask your doctor or pharmacist if you are unsure.
- It is important to use Symbicort Turbuhaler every day, even if you do not have any symptoms of asthma or COPD at the time.
- If you use Symbicort Turbuhaler for asthma, your doctor will check your symptoms regularly.
If you have been taking steroid tablets for asthma or COPD, your doctor will reduce the number of tablets you have to take when you start taking Symbicort Turbuhaler. If you have been taking steroid tablets for a long time, the doctor may want to take blood tests from time to time. When you reduce the number of tablets, you may generally feel worse even if your chest symptoms improve. You may get symptoms such as nasal congestion or runny nose, weakness, joint or muscle pain, or get a rash ( eczema ). If any of the symptoms become troublesome or if you experience symptoms such as headache, fatigue, nausea, or vomiting, contact your doctor immediately. You may need to change medication if you get symptoms of allergy or joint inflammation ( arthritis). Contact the doctor if you are worried about continuing to use Symbicort Turbuhaler.
Your doctor may consider adding steroid tablets to your usual treatment during stressful periods (for example, if you have a respiratory infection or before surgery).
Important information about your asthma or COPD symptoms
If you feel short of breath or wheezing while using Symbicort Turbuhaler, you should continue to use Symbicort Turbuhaler, but see a doctor as soon as possible as you may need further treatment.
Contact a doctor immediately if:
- Your breathing worsens or if you often wake up at night with asthma.
- You start to feel pressure on the chest in the morning or if the pressure on the chest lasts longer than usual.
These signs may indicate that your asthma or COPD is not adequately controlled and you may immediately need different or additional treatment.
Asthma
Symbicort Turbuhaler can be prescribed for asthma in two different ways. The amount of Symbicort Turbuhaler to use and when to use it depends on how the preparation has been prescribed for you.
- If you have been prescribed Symbicort Turbuhaler and a separate seizure suppressor, read the section called “a) Using Symbicort Turbuhaler and a separate seizure suppressor”.
- If you have been prescribed Symbicort Turbuhaler as your only inhaler, read the section called “b) Using Symbicort Turbuhaler as your only asthma inhaler”.
a) Using Symbicort Turbuhaler and a separate seizure suppressor
Use your Symbicort Turbuhaler every day. This helps prevent the onset of asthma symptoms.
Adults (18 years and older)
- The usual dose is 1 or 2 inhalations, twice a day.
- Your doctor may increase this to 4 inhalations, twice a day.
- If your symptoms are well controlled, your doctor may ask you to take the medicine once a day.
Youth (12 to 17 years)
- The usual dose is 1 or 2 inhalations, twice a day.
- If your symptoms are well controlled, your doctor may ask you to take the medicine once a day.
A lower strength of Symbicort Turbuhaler is available for children aged 6 to 11 years.
Symbicort Turbuhaler is not recommended for children under 6 years of age.
Your doctor (or asthma nurse) will help you manage your asthma. They will adjust the dose of one of the medicines to the lowest dose needed to control asthma. Do not adjust the dose or stop treatment without talking to your doctor (or asthma nurse) first.
Use your separate attack suppressor to treat asthma symptoms when they occur.
Always carry your raid chopper with you so you can use it when you need to. Do not use Symbicort Turbuhaler to treat your asthma attacks – use your attack suppressor.
b) Using Symbicort Turbuhaler as your only asthma inhaler.
Only use Symbicort Turbuhaler in this way if your doctor has told you to and only if you are 12 years of age or older.
Use your Symbicort Turbuhaler every day. This helps prevent the onset of asthma symptoms. You can take:
- 1 inhalation in the morning and 1 inhalation in the evening
or
- 2 inhalations in the morning
or
- 2 inhalations in the evening.
Your doctor may increase this to 2 inhalations twice a day.
Also use Symbicort Turbuhaler as a suppressor to treat asthma symptoms when they occur and to prevent the onset of asthma symptoms (for example, when you exercise or are exposed to allergens).
- If you get asthma symptoms, inhale 1 time and wait a few minutes.
- If you do not feel better, inhale again.
- Do not take more than 6 inhalations at one time.
Always carry your Symbicort Turbuhaler inhaler with you so you can use it when you need it.
A total daily dose of more than 8 inhalations is not normally needed. However, your doctor may tell you to take up to 12 inhalations per day for a limited period.
If you regularly need 8 inhalations or more in a day, see your doctor or nurse, they may need to change your treatment.
Do not use more than a total of 12 inhalations during 24 hours.
If you exercise and get asthma symptoms, use Symbicort Turbuhaler as described here. You must discuss the use of Symbicort Turbuhaler with your doctor to prevent asthma symptoms from occurring; how often you exercise and how often you are exposed to allergens can affect the treatment you are prescribed.
Chronic Obstructive Pulmonary Disease (COPD)
- For adults only (18 years and older).
- The normal dose is 2 inhalations twice a day.
Your doctor may also prescribe other bronchodilator drugs, such as anticholinergic drugs (such as tiotropium or ipratropium bromide), for your COPD.
Preparing your new Symbicort Turbuhaler inhaler
Before using your new Symbicort Turbuhaler inhaler for the first time, you need to prepare it for use as follows:
- Unscrew the protective sleeve and lift it off. You may hear a rattling sound.
- Hold your Symbicort Turbuhaler inhaler upright with the red knob facing down.
- Turn the red knob in one direction as far as it will go. Then turn it the other way as far as it will go (it doesn’t matter which way you turn first). You should hear a clicking sound. It doesn’t matter if the click comes on the first or second spin.
- Repeat this, turning the red knob in both directions.
- Your Symbicort Turbuhaler inhaler is now charged and ready to use.
How to inhale
Every time you need to do an inhalation, follow the instructions below.
1. Unscrew the protective sleeve and lift it off. You may hear a rattling sound.
2. Hold your Symbicort Turbuhaler inhaler upright with the red knob facing down.

3. Do not hold the mouthpiece when charging your Symbicort Turbuhaler inhaler. To feed a dose, turn the red knob in one direction as far as it will go.
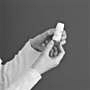
Then turn it the other way as far as it will go (it doesn’t matter which way you turn first). You should hear a clicking sound. It doesn’t matter if the click comes on the first or second spin. Your Symbicort Turbuhaler inhaler is now charged and ready to use. Only charge your Symbicort Turbuhaler inhaler when you need to use it.
4. Keep the Symbicort Turbuhaler inhaler away from the mouth. Exhale calmly (as much as feels comfortable). Do not exhale through your Symbicort Turbuhaler inhaler.
5. Carefully place the mouthpiece between the teeth. Close your lips. Breathe in through your mouth as deeply and forcefully as you can. Do not chew or bite the mouthpiece.

6. Remove the Symbicort Turbuhaler inhaler from your mouth. Then exhale gently. The amount of medicine that is inhaled is very small. This means that you may not feel any taste of it after inhaling it. If you have followed the instructions, you can still be sure that you have inhaled dose one and that the medicine is now in your lungs.
7. If you need to take another dose, repeat steps 2 to 6.
8. Replace the protective sleeve properly after use.

9. Rinse your mouth with water after your daily morning and/or evening doses, and spit out the water.
Do not attempt to remove or twist the nozzle. It is attached to your Symbicort Turbuhaler inhaler and must not be removed. Do not use your Symbicort Turbuhaler inhaler if it has been damaged or if the mouthpiece has come off your Symbicort Turbuhaler inhaler.
As with other inhalers, caregivers should ensure that children prescribed Symbicort Turbuhaler use the correct inhalation technique, as described above.
Cleaning your Symbicort Turbuhaler inhaler
Wipe the outside of the nozzle with a dry cloth once a week. Do not use water or other liquids.
When is it time to start using a new inhaler?
• The dose indicator tells you how many doses ( inhalations ) you have left in your Symbicort Turbuhaler inhaler, which is 30, 60, or 120 doses from the start when it is fully charged.

• The dose indicator is marked in intervals of 10 doses (for inhalers with 60 or 120 doses ) and intervals of 15 doses (for inhalers with 30 doses ). The dose indicator, therefore, does not show every dose.
• When you see a red mark at the edge of the indicator window, there are approximately 20 doses left. At the last 10 doses, the background of the dose indicator will turn red. When “0” with a red background appears in the middle of the indicator window, you must start on a new Symbicort Turbuhaler inhaler.
Note:
- The knob will still ‘click’ when you turn it even when your Symbicort Turbuhaler inhaler has run out.
- The sound you hear when you shake your Symbicort Turbuhaler inhaler comes from a drying agent and not from the medicine. Therefore, the sound cannot tell you how much medicine is left in your Symbicort Turbuhaler inhaler.
- If you accidentally charge your Symbicort Turbuhaler inhaler more than once before taking your dose, you will still only get one dose. However, the dose indicator will register all loaded doses.
If you have used too much Symbicort Turbuhaler
If you have ingested too much medicine or if, for example, a child has ingested the medicine by mistake, contact a doctor or hospital for an assessment of the risk and advice.
You must take the dose as stated on the label on the package or as your doctor has informed you. Do not exceed dose one without consulting a doctor.
The most common symptoms that can occur if you have used too much Symbicort Turbuhaler are tremors, headache, and rapid heartbeat.
If you forget to use Symbicort Turbohaler
- If you forget to take a dose, take it as soon as you remember. If, however, it is soon time for the next dose, skip the missed dose one.
- Do not take a double dose to make up for a missed dose.
If you have further questions about this medicine, contact your doctor or pharmacist.
Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
If any of the following happens, stop using Symbicort Turbuhaler and contact your doctor immediately:
- Swelling of the face, especially around the mouth (tongue and/or pharynx and/or difficulty swallowing) or hives together with difficulty breathing ( angioedema ) and/or a sudden feeling of fainting. This may mean that you have had an allergic reaction. This is rare, it happens to less than 1 in 1000 users.
- Sudden, acute wheezing or shortness of breath after inhaling the medicine. If any of these symptoms occur, stop using Symbicort Turbuhaler inhaler immediately and use your seizure suppressant. Contact your doctor immediately as you may need to change your treatment. This is very rare, occurring in fewer than 1 in 10,000 users.
Other possible side effects:
Common (may affect up to 1 in 10 users)
- Palpitations (you feel your heartbeat), tremors, or shaking. If these effects occur, they are usually mild and disappear as you continue to use Symbicort Turbuhaler.
- Fungal infection in the mouth. This is less likely to happen if you rinse your mouth with water after using your Symbicort Turbuhaler.
- A little sore throat, cough, and hoarse voice.
- Headache.
- Pneumonia in COPD patients.
Tell your doctor if you have any of the following symptoms while taking Symbicort Turbuhaler as they may be symptoms of pneumonia:
- fever or chills
- increased mucus production changed the color of the mucus
- increased coughing or increased breathing difficulties
Uncommon (may affect up to 1 in 100 users)
- Feeling restless, nervous, or agitated.
- Disturbed sleep.
- Dizziness.
- Nausea.
- Rapid heartbeat.
- Bruises on the skin.
- Muscle cramps.
- Blurred vision.
Rare (may affect up to 1 in 1,000 users)
- Rash, itching.
- Tracheal spasm (contraction of the muscles in the airways, causing wheezing). If wheezing occurs suddenly after using Symbicort Turbuhaler, stop using Symbicort Turbuhaler and consult a doctor immediately.
- Low levels of potassium in the blood.
- Irregular heartbeat.
Very rare (may affect up to 1 in 10,000 users)
- Depression.
- Behavioral changes, especially in children.
- Chest pain or pressure in the chest (angina).
- An increased amount of sugar ( glucose ) in the blood.
- Taste changes, such as an unpleasant taste in the mouth.
- Changes in blood pressure et.
Inhaled corticosteroids can affect the normal production of steroid hormones in the body, especially if you use high doses for a long time. The effects include:
- Changes in bone mineral density (thinning of the skeleton).
- Cataracts (clouding of the lens of the eye).
- Green cataract (increased pressure in the eye).
- A reduced growth rate in children and adolescents.
- An effect on the adrenal glands (a small gland that sits next to the kidney).
The likelihood of these effects occurring is much less with inhaled corticosteroids than with cortisone tablets.
How to store Symbicort Turbuhaler
- Keep this medicine out of the sight and reach of children.
- Use before the expiry date which is stated on the carton or label of the inhaler after EXP. The expiration date is the last day of the specified month.
- No special storage instructions.
- Medicines must not be thrown into the drain or among the household waste. Ask the pharmacist how to dispose of medicines that are no longer used. These measures will help to protect the environment.
Contents of the packaging and other information
Contents declaration
The active substances are budesonide and formoterol fumarate dihydrate. Each inhaled dose contains 160 micrograms of budesonide and 4.5 micrograms of formoterol fumarate dihydrate.
Other ingredients are lactose monohydrate (which contains milk proteins).
Appearance and package sizes of the medicine
Symbicort Turbuhaler is an inhaler that contains medicine. The color of the inhalation powder is white. Each inhaler contains either 30, 60, or 120 doses and is white with a red knob. The dial is brailled with the number 6 for identification, to distinguish it from other AstraZeneca inhalation products.
Symbicort Turbuhaler is available in packs of 1 inhaler containing 30 doses or in packs of 1, 2, 3, 10, or 18 inhaler(s) each containing 60 or 120 doses. Not all pack sizes may be marketed.
This medicine is approved in the European Economic Area under the names:
Austria : Symbicort Turbohaler 160 μg/4.5 μg/inhalation; Belgium : Symbicort Turbohaler 160 μg/4.5 μg/inhalation; Bulgaria : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Croatia : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Cyprus : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Czech Republic : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Denmark : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Estonia : Symbicort Turbuhaler , 160 μg/4.5 μg;Finland : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; France : Symbicort Turbuhaler 200 μg/6 μg/inhalation; Germany : Symbicort Turbohaler 160 μg/4.5 μg/inhalation; Greece : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Hungary : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Iceland : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Ireland : Symbicort Turbohaler 200 μg/6 μg/inhalation; Italy : Symbicort 160 μg/4.5 μg/inhalation; Latvia: Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Lithuania : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Luxembourg : Symbicort Turbohaler 160 μg/4.5 μg/inhalation; Malta : Symbicort Turbohaler 200 μg/6 μg/inhalation; The Netherlands : Symbicort Turbuhaler 200 μg/6 μg/inhalation; Norway : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Poland : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Portugal : Symbicort Turbohaler 160 μg/4.5 μg/inhalation;Romania : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Slovakia : Symbicort Turbuhaler 200 μg/6 μg/inhalation; Slovenia : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Spain : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; Sweden : Symbicort Turbuhaler 160 μg/4.5 μg/inhalation; United Kingdom : Symbicort Turbohaler 200 μg/6 μg/inhalation.