Oculac 50 mg/ml Eye Drops Povidone K25 Solution
What Oculac Is And What It Is Used For
The active substance in Oculac is called povidone K25. Oculac eye drops are for moisturizing and lubricating the eyes. The drops are used to relieve problems caused by dry eyes.
What You Need To Know Before You Use Oculac
Do Not Use Oculac
if you are allergic to povidone K25 or any of the other ingredients of this medicine (see section 6). If you think you may be allergic, do not use this medicine until you have consulted a doctor.
Warnings And Cautions
Talk to your doctor or pharmacist before using Oculac eye drops.
If you experience
- Headache
- Eye pain
- Vision changes
- Eye irritation
- Persistent redness, or if the condition persists or worsens – discontinue treatment and consult a physician.
Other Medicines And Oculac
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines.
If you are taking other ophthalmic medicines with Oculac, you should take a break of at least 5 minutes between the different medicines and always apply Oculac last.
Pregnancy And Breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine. Oculac can be used during pregnancy and lactation.
Driving And Using Machines
Oculac can make your field of vision cloudy (cause blurred vision). If this happens to you, wait until the field of vision is clear again before driving or using machines.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires sharpened vigilance. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. Descriptions of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
Oculac Contains Benzalkonium Chloride
- This medicine contains 0.0018 mg benzalkonium chloride per drop equivalent to 0.05 mg/ml.
- Benzalkonium chloride can be absorbed by soft contact lenses and may discolor the contact lenses. Remove contact lenses before using the medicine and wait at least 15 minutes before re-inserting the contact lenses.
- Benzalkonium chloride can be irritating to the eyes, especially if you have dry eyes or problems with the cornea (the clear membrane at the front of the eye). If you experience irritation, tingling, or pain in the eye after using the medicine, consult a doctor.
How To Use Oculac
Oculac eye drops uses
- Always use this medicine exactly as described in this leaflet or as your doctor or pharmacist has told you. Ask your doctor or pharmacist if you are unsure.
- A typical dose is 1 drop in each eye 4 times daily or as needed.
- If a drop misses the eye, try again.
- If you use more Oculac eye drops than you should, no side effects are expected.
- If you use other eye drops or ointments, wait at least 5 minutes between each medicine. Oculac should be applied last.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Instructions for use
- Wash your hands.
- Open the bottle. Do not touch the tip of the bottle after opening it, as this may contaminate the solution.
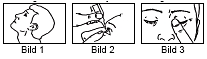
- Bend your head back (Figure 1).
- Pull the lower eyelid down with one finger and hold the drip bottle in your other hand. The tip of the bottle must not touch the eye, as this may damage the eye. Press the bottle so that a drop falls into the eye. (Picture 2).
- Close the eye and press a fingertip against the inner corner of the eye for 1-2 minutes. This prevents the drop from flowing through the tear duct into the pharynx, and means that a larger part of the drop remains in the eye (Fig. 3). If necessary, repeat steps 3-5 for your other eye.
- Close the bottle after each use.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
Possible Povidone k25 Side Effects
Like all medicines, Oculac can cause side effects, although not everybody gets them.
The following side effects have been reported:
Common (may affect up to 1 in 10 people):
- Mild
- Transient burning or sticky sensation in the eye
Very rare (may affect up to 1 in 10,000 people):
- Irritation
- Hypersensitivity reaction er
Has been reported (occurs in an unknown number of users):
- Blurred vision
- Eye pain
- Itching
- Redness of the eyes
How To Store Oculac
- Keep out of sight and reach of children.
- Store at a maximum of 25 o C. Store in protection against cold. Do not freeze.
- Store in the outer carton. Sensitive to light.
- The bottle itself with eye drops is not sterile, but the contents of the bottle retain their sterility until the bottle is opened. The eye drops must then be used within 4 weeks after the bottle has been opened for the first time.
- Do not use this medicine after the expiry date which is stated on the carton and bottle (EXP). The expiration date is the last day of the specified month.
The medicine should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
Contents Of The Pack And Other Information
Content Declaration
Oculac eye drops contain povidone K25 as an active substance. Each milliliter contains 50 mg of povidone K25. The other ingredients are benzalkonium chloride ( preservative ), boric acid, calcium chloride, potassium chloride, magnesium chloride, sodium chloride, sodium lactate sodium hydroxide (for pH adjustment), and water for injections.
What The Medicine Looks Like And Contents Of The Pack
- Description of Oculac eye drops slightly yellowish, clear aqueous solution.
- Pack size: 10 ml plastic bottle
Marketing Authorisation Holder
Laboratoires THEA, 12, rue Louis Blériot, 63017 Clermont-Ferrand Cedex 2, France
Manufacturer
EXCELVISION, 29 rue de la Lombardière, 07100 Annonay, France
SA Alcon- Couvreur NV, Rijksweg 14, 2870 Puurs, Belgium
Alcon Laboratories Belgium, Lichterveld 3, 2870 Puurs-Sint-Amands, Belgium
This medicinal product is authorized under the European Economic Area under the names:
Denmark Oculac (MDU)
Finland Oculac 50 mg/ml silmätipat, liuos
Portugal Oculotect (fluid)
Spain Oculotect 50 mg/ml colirio in solution