0.5 mg / 2.5 mg solution for nebulizer
ipratropium bromide and salbutamol
What Ipratropium / Salbutamol Sandoz is and what it is used for
This medicine is called Ipratropium / Salbutamol Sandoz. The active substances are ipratropium bromide and salbutamol . Ipratropium bromide and salbutamol both belong to the group of bronchodilators and make it easier for you to breathe by opening up the airways. This is done by the drug preventing the smooth muscles surrounding the airways from contracting, thus keeping the airways open. Ipratropium bromide works by blocking nerve signals to the muscles around the airways. Salbutamol acts by stimulating the beta 2 – receptor in your muscles.
Ipratropium / Salbutamol Sandoz is used to treat breathing problems in people with long-term breathing difficulties (eg chronic bronchitis or emphysema ). Ipratropium / Salbutamol Sandoz provides relief from wheezing, shortness of breath, and tightness in the chest.
Ipratropium bromide and salbutamol contained in Ipratropium / Salbutamol Sandoz may also be approved for the treatment of other conditions not mentioned in this product information. Ask your doctor, pharmacist, or another healthcare professional if you have any further questions, and always follow their instructions.
What you need to know before you use Ipratropium / Salbutamol Sandoz
Do not use Ipratropium / Salbutamol Sandoz if:
- You are allergic to ipratropium or
- salbutamol or any of the other ingredients of this medicine (listed in section 6).
- You are allergic to similar medicines containing atropine or atropine-like medicines.
- You have a fast heartbeat ( tachyarrhythmia ).
- You have enlarged heart or the disease hypertrophic obstructive cardiomyopathy .
Warnings and cautions
Talk to your doctor or pharmacist before using Ipratropium / Salbutamol Sandoz if:
- You experience abnormal muscle cramps around the trachea (paradoxical bronchospasm ) after inhaling ipratropium / salbutamol. This can be life threatening and therefore you must stop using this medicine immediately. Contact your doctor immediately so that replacement therapy can be instituted.
- You have difficulty regulating your diabetes .
- You have or have recently had problems with your heart or blood circulation.
- You feel pressure over your chest.
- You have hyperthyroidism ( hyperthyroidism ).
- You have a rare tumor in the adrenal medulla ( pheochromocytoma ).
- You have glaucoma (increased eye pressure) or have been told that you can get glaucoma . Your doctor may recommend that you protect your eyes when using Ipratropium / Salbutamol Sandoz, e.g. by using a nozzle.
- You have an enlarged prostate .
- You have difficulty emptying your bladder.
- You have cystic fibrosis and thus may have indigestion.
A condition called lactic acidosis (increased acidity ( lactic acid ) in the blood) has been reported with high doses of salbutamol, especially in patients being treated for acute bronchospasm (tracheal spasm) (see sections 3 and 4). An increase in lactate levels (lactic acid level) can lead to difficulty breathing and hyperventilation (rapid and shallow breathing) even if other symptoms such as wheezing improve. If you experience that your medicine does not work as well as usual and you need to use the nebulizer more than your doctor has prescribed, talk to a doctor immediately.
Other medicines and Ipratropium / Salbutamol Sandoz
Tell your doctor or pharmacist if you are taking, have recently taken, or might take any other medicines, especially with any of the following medicines:
- other drugs for respiratory diseases, including bronchodilators such as beta 2 immimetics, anticholinergics and xanthine derivatives (eg theophylline). These may increase the effect of Ipratropium / Salbutamol Sandoz and make the side effects more severe.
- certain medicines for high blood pressure ( beta blockers )
- certain medicines for depression (such “antidepressants” are medicines that are prescribed to patients suffering from depression and anxiety). This class of drugs includes, for example, monoamine oxidase inhibitors (eg phenelzine) and tricyclic antidepressants (eg amitriptyline).
- digoxin (for heart problems) can cause heart rhythm problems when taken at the same time as Ipratropium / Salbutamol Sandoz
- diuretics (so-called diuretics ) can cause an inability to empty the bladder
- steroid tablets (medicines used to reduce inflammation in your body, such as prednisolone ), which may aggravate airway obstruction
- Anesthetics can increase the heart’s sensitivity to salbutamol . If you are going to have surgery, you will be closely monitored. Your doctor may decide to stop treatment with Ipratropium / Salbutamol Sandoz if you are having surgery. If you are going to be anesthetized in a hospital, tell the anesthetist which medicines you are using.
Pregnancy and breastfeeding
If you are pregnant or breastfeeding, think you may be pregnant, or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Ipratropium / Salbutamol Sandoz should only be used during pregnancy or breastfeeding after discussing this with your doctor.
Talk to your doctor before taking any medicine.
Driving and using machines
If you experience side effects such as dizziness, difficulty focusing, and blurred vision when treated with Ipratropium / Salbutamol Sandoz, you should avoid doing things that may involve risks, e.g. to drive a car or use machines.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires sharpened attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. Descriptions of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
How to take Ipratropium / Salbutamol Sandoz
Always use this medicine exactly as your doctor or pharmacist has told you. Ask your doctor or pharmacist if you are unsure.
Ipratropium / Salbutamol Sandoz is for inhalation use. The nebulizer solution is intended for inhalation by mouth after nebulization.
The recommended dose for adults and children over 12 years is a single-dose container three or four times a day.
Elderly patients should take the dose recommended for adults.
Ipratropium / Salbutamol Sandoz is not recommended for use in children below 12 years of age.
How much to take and how often is stated on the label.
Never take more medicine than your doctor has prescribed. Tell your doctor if your breathing problems get worse or if the medicine does not provide the same relief for your breathing problems as before, or if you use the blue fast-acting inhaler more often than usual.
Ipratropium / Salbutamol Sandoz should be used with a suitable nebulizer, e.g. a PARI LC PLUS nebulizer or jet nebulizer. Read the complete instructions for use for the nebulizer in the information supplied with the PARI LC PLUS before you start inhaling.
Instructions for use
- Prepare the nebulizer for use according to the manufacturer’s instructions and the doctor’s recommendations.
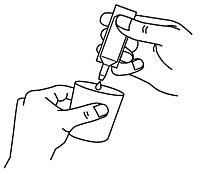
- Carefully remove a single-dose container from the labeled strip by twisting and pulling. Never use a single-dose container that has already been opened or if the nebuliser solution is discolored (Fig. A).
- Hold the single-dose container upright and turn off the lid (Fig. B).
- Squeeze the contents of the chamber into your nebulizer (Figure C).
- Follow the manufacturer’s instructions and your doctor’s advice on how to assemble and use the nebulizer.
- After using the nebulizer, discard any nebulizer solution left in the chamber. The nebuliser solution remaining in the single-dose container should also be discarded.
- Thoroughly clean the nebulizer according to the manufacturer’s instructions. It is important that the nebulizer is kept clean.
Do not dilute the nebulizer solution or mix it with other medicines unless your doctor tells you to.
The single-dose containers of Ipratropium / Salbutamol Sandoz do not contain preservatives. Therefore, it is important that the content is used immediately after opening. You must take a new container each time you use Ipratropium / Salbutamol Sandoz in your nebulizer.
Partially used, opened, or damaged single-dose containers should be discarded. Never use a single-dose container that has been opened before.
It is important to follow these instructions to avoid contaminating the nebulizer solution in the single-dose container.
Swallow not the nebulizer solution and use it for injection.
Do not allow the nebulizer solution or aerosol to enter the eyes.
If you use more Ipratropium / Salbutamol Sandoz than you should
If you have taken a slightly larger dose than usual, you may feel your heartbeat faster (palpitations) or start shaking. Other symptoms may include chest pain, altered blood pressure, redness, restlessness, or dizziness. These symptoms usually go away within a few hours. The amount of potassium in the blood may decrease and the doctor may want to check the potassium content of the blood by taking blood samples from time to time and measuring the level. Tell your doctor if you are worried about any of these symptoms or if they do not go away.
If you have ingested too much medicine or if e.g. a child ingested the medicine by mistake, contact a doctor, hospital, or the Poison Information Center for risk assessment and advice. Take all your medicines with you to the doctor or hospital, even those that are over-the-counter. If possible, they should remain in their original packaging. Bring this leaflet and show it to your doctor.
If you forget to use Ipratropium / Salbutamol Sandoz
If you forget to take a dose at the right time, take it as soon as you remember. Do not take a double dose to make up for a forgotten dose.
If you stop taking Ipratropium / Salbutamol Sandoz
Do not stop taking Ipratropium / Salbutamol Sandoz without talking to your doctor first.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Serious side effects are
If your asthma or wheezing worsens immediately after inhaling Ipratropium / Salbutamol Sandoz, or if it becomes more difficult for you to breathe and you become short of breath, do not take more Ipratropium / Salbutamol Sandoz without using your fast-acting inhaler immediately. Stop using Ipratropium / Salbutamol Sandoz and contact your doctor immediately. Your doctor may prescribe another treatment for your illness.
If you experience pain or discomfort in your eyes, blurred or red eyes, or if you see auras (light rings) or colored dots, contact your doctor immediately as you may need treatment for these symptoms.
If you think you may be allergic to Ipratropium / Salbutamol Sandoz or you may have had an allergic reaction to the nebulizer solution, stop using Ipratropium / Salbutamol Sandoz immediately and contact a doctor immediately.
The salbutamol component of Ipratropium / Salbutamol Sandoz may cause a decrease in the amount of potassium in the blood ( hypokalaemia ). This can cause muscle weakness, twitching, and abnormal heart rhythm. The probability of this happening is greater if you take Ipratropium / Salbutamol Sandoz with any other asthma treatment with inhaled steroid you, steroid pill, or diuretics. Your doctor may need to take blood samples to test your potassium levels from time to time.
Side effects can occur with the following frequency:
Uncommon (may affect up to 1 in 100 people):
- nervousness
- headache
- shaking, trembling
- dizziness
- palpitation
- fast heartbeat
- high (upper) blood pressure
- cough
- huskiness
- dry mouth
- nausea
- irritation of the pharynx
- altered sense of taste
- skin reactions.
Rare (may affect up to 1 in 1,000 people):
- severe, life-threatening allergic reaction to certain substances ( anaphylactic reaction with swelling that may affect the tongue, lips and face)
- low potassium levels in the blood ( hypokalaemia )
- mental disorders
- difficulty focusing the gaze
- visual disturbances
- elevated intraocular pressure ( glaucoma )
- enlarged pupils ( mydriasis )
- dimsyn
- eye pain
- Red eyes
- presence of colored rings around points of light in the field of view (halofenomen)
- abnormal or very fast heart rhythm
- heart attack
- low (lower) blood pressure
- dryness in the throat
- breathing or speech difficulties due to short-term muscle cramps in the airways or pharynx
- inflammation of the mouth and / or pharynx and / or accumulation of fluid in the mouth and / or pharynx
- diarrhea
- vomiting
- constipation
- indigestion
- caries
- rash, hives
- itching
- excessive sweating
- muscle cramps, weakness and / or pain
- difficulty emptying the bladder
- weakness.
Has been reported (occurs in an unknown number of users):
- a condition called lactic acidosis (increased acidity ( lactic acid )) that can cause abdominal pain, hyperventilation (rapid and shallow breathing), difficulty breathing (although other symptoms such as wheezing can be improved), cold hands and feet, irregular heartbeat or thirst.
Some people can get chest pain (due to angina), but it is not known exactly how often this happens. Tell your doctor as soon as possible if you get these symptoms while you are being treated with Ipratropium / Salbutamol Sandoz, but do not stop taking this medicine unless your doctor tells you to.
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist, or nurse. This also applies to any side effects not mentioned in this information. You can also report side effects directly to the Medical Products Agency. By reporting side effects, you can help increase drug safety information.
5. How to store Ipratropium / Salbutamol Sandoz
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiration date is the last day of the specified month.
Store in a cold place. Do not freeze. Do not store above 25 ° C.
Store the nebulizer solution in the outer bag or carton. Sensitive to light.
After the first opening of the outer bag, the single-dose containers should be used within 3 months.
For single use only. Use immediately after opening the single-dose container.
Discard immediately after first use.
Partially used, opened, or damaged single-dose containers should be disposed of in accordance with local requirements.
Do not use this medicine if you notice that the nebulizer solution is cloudy.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the packaging and other information
Content declaration
Each 2.5 ml single-dose container of Ipratropium / Salbutamol Sandoz contains:
- The active substance is 0.5 mg ipratropium bromide corresponding to 0.520 mg ipratropium bromide monohydrate and 2.5 mg salbutamol as sulphate.
- The other ingredients are sodium chloride, water for injections and sulfuric acid.
What the medicine looks like and contents of the pack
The single-dose container is an FFS‑ (Plastic Form fill Seal) ampoule containing 2.5 ml of nebulizer solution packaged in a three-layer foil pouch containing five single-dose containers in each and then in cardboard.
Multiple packs containing 10 single-dose containers (2 bags with 5 single-dose containers ), 20 single-dose containers (4 bags with 5 single-dose containers ), 30 single-dose containers (6 bags with 5 single-dose containers ), 40 single-dose containers (8 bags with 5 single-dose containers ), 50 single-dose containers (10 bags with 5 single-dose containers ), 60 single-dose containers (12 bags with 5 single-dose containers ), 80 single-dose containers (16 bags with 5 single-dose containers ), 100 single-dose containers (20 bags with 5 single-dose containers), 120 single-dose containers (24 bags with 5 single-dose containers ) and 150 single-dose containers (30 bags with 5 single-dose containers ).
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorisation Holder:
Sandoz A / S, Edvard Thomsens Vej 14, 2300 Copenhagen S, Denmark
Manufacturer:
Salutas Pharma GmbH, Otto-von-Guericke-Allee 1, 39179 Barleben, Germany