E
2,000 IU / ml, 4,000 IU / ml, 10,000 IU / ml, 40,000 IU / ml solution for injection in pre-filled syringe
epoetin alfa
1. What Eprex is and what it is used for
Eprex contains the active substance epoetin alfa – a protein that stimulates the bone marrow to produce more red blood cells that contain hemoglobin (a substance that carries oxygen). Epoetin alfa is a copy of the human protein et erythropoietin (e-right-topo-e-tin) and works in the same way.
The erythropoietin contained in Eprex may also be approved for the treatment of other conditions not mentioned in this leaflet. Ask your doctor, pharmacist, or another healthcare professional if you have any further questions, and always follow their instructions.
- Eprex is used to treat symptomatic anemia ( anemia ) caused by kidney disease:
- in children undergoing hemodialysis
- in adults undergoing hemodialysis or peritoneal dialysis
- in adults with severe anemia who have not yet undergone dialysis
If you have kidney disease and your kidney does not produce enough erythropoietin, you may have too few red blood cells. Erythropoietin is needed for the production of red blood cells. Eprex stimulates your bone marrow to produce more red blood cells.
- Eprex is used to treat anemia in adults receiving chemotherapy for solid (solid) tumor s, lymph node tumors (malignant lymphoma ), or multiple myeloma (bone marrow cancer), and which may need a blood transfusion. Eprex may reduce the need for a blood transfusion in these patients.
- Eprex is used in adults with moderate anemia who donate a certain part of their blood before their operation so that they can get it back during or after the operation. Because Eprex stimulates the production of red blood cells, a larger volume of blood can be taken from these people.
- Eprex is used in people with moderate anemia who need major orthopedic surgery ( eg hip and knee surgery ) to reduce the need for blood transfusions
- Eprex is used to treat anemia in adults with a bone marrow disease that causes a serious disorder in the formation of blood cells ( myelodysplastic syndrome ). Eprex may reduce the need for a blood transfusion.
2. What you need to know before you use Eprex
Do not use Eprex
- If you are allergic to epoetin alfa or any of the other ingredients of this medicine (listed in section 6).
- If you have been diagnosed with erythroblastopenia (meaning that your bone marrow cannot produce enough red blood cells ) after previous treatment with any product that stimulates the formation of red blood cells (including Eprex). See section 4, Possible side effects are.
- If you have high blood pressure that is not adequately controlled with medication.
- To stimulate the production of your red blood cells (so that doctors can take a larger volume of blood from you) if you can not get a transfusion with your own blood during or after the operation.
- If you are going to undergo major planned orthopedic surgery (eg hip or knee surgery) and:
- have severe heart disease
- serious diseases of the veins and arteries you
- have recently had a heart attack or stroke ( stroke )
- can not take blood thinners.
Eprex may be inappropriate for you. Discuss with your doctor. Some people taking Eprex may also need medicines that reduce the risk of blood clots (blood thinners). If you can not take blood-thinning medicines, do not use Eprex.
Warnings and cautions
Take special care with Eprex
Eprex and other products that stimulate the formation of red blood cells may increase the risk of developing blood clots in all patients. This risk may be higher if you have other risk factors for developing blood clots (eg if you have had a blood clot before or if you are overweight, have diabetes, heart disease, or are in bed for a long time due to surgery or illness). Tell your doctor if you have any of these risk factors. Your doctor will decide if Eprex is right for you.
You must tell your doctor if any of the following apply to you. You may still be able to use Eprex, but discuss it with your doctor first.
- If you know you are suffering from or have suffered from:
- high blood pressure
- epileptic seizures
- liver disease
- anemia for other reasons
- porphyria (a rare blood disorder)
- If you are a patient with chronic kidney failure, and especially if you do not respond adequately to Eprex, your doctor will check your dose of Eprex, as repeated dose increases of Eprex if you do not respond to treatment may increase the risk of heart or blood vessel problems and may increase risk of heart attack, stroke ( stroke ) and death.
- If you are a cancer patient, you should be aware that products that stimulate the formation of red blood cells such as Eprex can act as a growth factor and could therefore theoretically affect the development of your cancer. Depending on your condition, a blood transfusion may be preferable. Discuss this with your doctor.
- If you are a cancer patient, you should be aware that the use of Eprex may be associated with shorter survival and higher mortality in head and neck cancer as well as metastatic breast cancer in patients treated with chemotherapy.
- Serious skin reactions such as Steven-Johnson syndrome and toxic epidermal necrolysis have been reported during treatment with epoetin.
Steven-Johnson syndrome and toxic epidermal necrolysis can begin as reddish-purple target-like or round spots on the torso, often with blisters in the middle. Sores in the mouth, throat, nose, genitals, and eyes (red and swollen eyes) may also occur. These severe skin reactions are often preceded by fever and/or flu-like symptoms. The rash can develop into widespread areas with scaly skin and life-threatening complications.
If you get a severe rash or any of these skin symptoms, stop taking Eprex and contact a doctor or hospital immediately.
Take special care with other products that stimulate the formation of red blood cells:
Eprex belongs to a group of products that stimulate the formation of red blood cells, as does the human protein et erythropoietin. The healthcare staff will always register (note in the medical record) exactly which product you are using. If you get any product in this group other than Eprex during your treatment, talk to your doctor or pharmacist before using it.
Other medicines and Eprex
Tell your doctor if you are taking, have recently taken, or might take any other medicines.
If you are taking the drug ciclosporin (used, for example, after a kidney transplant), your doctor may prescribe blood tests to check your ciclosporin levels while you are taking Eprex.
Iron supplements and other blood stimulants can increase the effect of Eprex. Your doctor will decide if you should take such.
If you visit a hospital or go to your clinic or GP, tell them that you are being treated with Eprex. It may affect other treatments or test results.
Pregnancy and breastfeeding
You must tell your doctor if any of the following apply to you. You may still be able to take Eprex but discuss with your doctor first.
- If you are pregnant or think you may be pregnant.
- If you are breast-feeding.
Driving and using machines
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and/or side effects. Descriptions of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
Eprex contains sodium
This medicine contains less than 1 mmol sodium (23 mg) per dose, ie essentially ‘ sodium-free’. next to “sodium-free”.
3. How to use Eprex
Always use this medicine exactly as your doctor has told you. Consult a doctor if you are unsure.
Your doctor has taken blood samples and decided that you need Eprex.
Eprex is given by injection:
- either in a vein or in a tube that enters a vein ( intravenously )
- or under the skin ( subcutaneously ).
Your doctor will decide how to inject Eprex. You will usually receive the injections from a doctor, nurse, or other healthcare professional. Depending on why they need Eprex treatment, some people may later learn to inject themselves under the skin: see Instructions on how to inject Eprex yourself.
Eprex should not be used:
- after the expiry date which is stated on the label and the outer carton
- if you know or believe that it may have been accidentally frozen, or
- if the refrigerator has stopped working.
Your Eprex- dose is based on your body weight in kilograms. The cause of your anemia is also included as a factor when your doctor decides which dose is right for you.
Your doctor will monitor your blood pressure regularly while you are taking Eprex.
People with kidney disease
- Your doctor will keep your hemoglobin level between 100 and 120 g / l, as high hemoglobin levels may increase the risk of blood clots and death. In children, the hemoglobin level should be kept between 95 and 110 g / l.
- The usual starting dose of Eprex for adults and children is 50 international units (IU) per kilogram (/ kg) of body weight given three times a week.
- For patients on peritoneal dialysis, Eprex can be given twice a week.
- For adults and children, Eprex is given as an injection either into a vein or into a tube that goes into a vein. When this entry (via a vein or tube) is not easily accessible, your doctor may decide that Eprex should be injected under the skin ( subcutaneously ). This includes patients undergoing dialysis and patients not yet undergoing dialysis.
- Your doctor will prescribe regular blood tests to see how your anemia responds to treatment and may adjust dose one, usually no more often than every four weeks. An increase in hemoglobin of more than 20 g / l over a period of four weeks should be avoided.
- After your anemia has improved, your doctor will regularly continue to check your blood. Your Eprex dose and frequency of administration can be further adjusted to maintain your response to treatment. Your doctor will use the lowest effective dose to keep the symptoms of your anemia under control.
- If you do not respond well enough to Eprex, your doctor will check your dose and tell you if you need to change the dose of Eprex.
- If you are on a longer dosing interval of Eprex (less frequently than once a week), you may not be able to maintain adequate hemoglobin levels and you may need to increase the Eprex dose or the frequency of administration.
- You may receive iron supplements before and during Eprex treatment to make it more effective.
- If you are undergoing dialysis treatment when you start treatment with Eprex, your dialysis treatment may need to be adjusted. Your doctor will decide this.
Adults undergoing chemotherapy
- Your doctor may start treatment with Eprex if your hemoglobin level is 100 g / l or lower.
- Your doctor will keep your hemoglobin level between 100 and 120 g / l, as high hemoglobin levels may increase the risk of blood clots and death.
- The starting dose is either 150 IU per kilogram of body weight three times a week or 450 IU per kilogram of body weight once a week.
- Eprex is given by injection under the skin.
- Your doctor will prescribe blood tests and may adjust the dose depending on how your anemia responds to Eprex treatment.
- You may receive iron supplements before and during Eprex treatment to make it more effective.
- You will usually continue your Eprex treatment for one month after the end of chemotherapy.
Adults who donate their own blood
- The usual dose is 600 IU per kilogram body weight twice a week.
- Immediately after donating blood, Eprex is given by injection into a vein for 3 weeks before your operation.
- You may receive iron supplements before and during Eprex treatment to make it more effective.
Adults undergoing major orthopedic surgery
- The recommended dose is 600 IU per kilogram of body weight once a week.
- Eprex is given by injection under the skin every week for three weeks before the operation and on the day of the operation.
- If there is a medical need to reduce the time until your surgery, you will receive a daily dose of 300 IU / kg for up to ten days before the operation, on the day of the operation, and for four days immediately thereafter.
- If blood tests show that your hemoglobin is too high before the operation, treatment will be stopped.
- You may receive iron supplements before and during Eprex treatment to make it more effective.
Adults with myelodysplastic syndrome
- Your doctor may start treatment with Eprex if your hemoglobin level is 100 g / l or lower. The treatment aims to keep the hemoglobin level between 100 and 120 g / l, as a higher hemoglobin level can increase the risk of blood clots and deaths.
- Eprex is given by injection under the skin.
- The starting dose is 450 IU per kilogram of body weight once a week.
- Your doctor will prescribe blood tests and may adjust the dose depending on how your anemia responds to Eprex treatment.
Instructions on how to inject Eprex yourself
When treatment starts, Eprex is usually injected by healthcare professionals. Later, your doctor may suggest that you or your caregiver learn how to inject Eprex under the skin ( subcutaneously ).
- Do not try to give yourself an injection unless your doctor or nurse has trained you to do so.
- Always use Eprex exactly as your doctor or nurse has told you.
- Eprex should only be used if it has been stored correctly – see section 5, How to store Eprex.
- Before use, place the Eprex syringe until it reaches room temperature. This usually takes between 15 and 30 minutes.
Take only one dose of Eprex from each syringe.
If Eprex is injected under the skin ( subcutaneously ), the amount injected is usually no more than one milliliter (1 ml) in one injection.
Eprex is given alone and should not be mixed with other solutions for injection.
Do not shake the Eprex syringe. Violent shaking for a long time can damage the product. If the product has been heavily shaken, it must not be used.
How to give yourself an injection with a pre-filled syringe
The pre-filled syringes are equipped with a PROTECTS needle protection device, so that puncture damage after use can be prevented. This is stated on the packaging.
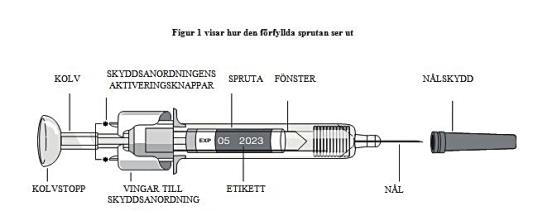
- Take a syringe out of the refrigerator. Allow the liquid to reach room temperature. Do not remove the needle cover from the syringe while it is at room temperature.
- Check the syringe to see that it contains the correct dose, that the expiry date has not passed, that it is not damaged, and that the liquid is clear and not frozen.
- Choose an injection site. Good places are on the top of the thigh and around the abdomen but not near the navel. Vary the injection site from day today.
- Wash your hands. Use an antiseptic wipe at the injection site to disinfect it.
- Hold the pre-filled syringe with the covered needle pointing upwards.
- Do not hold the piston stop, the piston, the wings of the needle guard, or the needle guard
- Do not pull the plunger back at any time
- Do not remove the needle cover from the pre-filled syringe until you are ready to inject your Eprex
- Remove the cover from the syringe by holding the syringe and gently pulling off the cover without twisting it. Do not touch the needle or shake the syringe. If, according to your doctor’s prescription, you only need a portion of dose one in the syringe, push the plunger to the desired numbered graduation mark to remove excess solution before injection.
- Do not touch the actuator activation buttons (indicated by asterisks * in Figure 1) to prevent the needle from being covered prematurely
- Pinch a skin fold between your thumb and forefinger. Do not squeeze.
- Push the needle down completely. Your doctor or nurse may have shown you how to do this.
- Press the plunger with your thumb as far as it will go to inject the entire amount of liquid. Press it in slowly and evenly with a preserved grip on the skin fold. The PROTECS needle protection device is not activated until the full dose has been given. You can hear a click when the PROTECS needle protection device has been activated.
- When the plunger has been pushed in as far as it will go, remove the needle and release the skin.
- Slowly release the plunger with your thumb and allow the syringe to pull upwards until the needle is fully retracted into the PROTECS protection device.
- When the needle is pulled out of your skin, it may bleed a little from the injection site. It’s normal. You can press an antiseptic wipe over the injection site for a few seconds after injection one.
- Dispose of the used syringe in a safe container – see section 5, How to store Eprex.
If you use more Eprex than you should
Tell your doctor or nurse immediately if you think too much Eprex has been injected. Side effects from an overdose of Eprex are unlikely.
If you have ingested too much medicine or if e.g. If a child has inadvertently ingested the medicine, contact a doctor, hospital, or the Poison Information Center for risk assessment and advice.
If you forget to take Eprex
Take the next injection as soon as you remember. If it is less than a day until your next injection , you can skip the one you missed and continue according to your usual schedule. Do not give double injection to replace the missed dose .
If you have hepatitis C and are being treated with interferon and ribavirin
Talk to your doctor as concomitant treatment with epoetin alfa and interferon and ribavirin may lead to loss of effect and, in rare cases, the development of a condition called erythroblastopenia (PRCA), a severe form of anemia . Eprex is not approved for the treatment of anemia associated with hepatitis C.
If you have any further questions on the use of this product, ask your doctor, nurse or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Tell your doctor or nurse immediately if you notice any of the following side effects .
Severe skin rashes such as Steven-Johnson syndrome and toxic epidermal necrolysishave been reported during treatment with epoetin. These conditions can cause symptoms such as reddish-purple target-like or round spots on the torso, often with blisters in the middle, scaly skin, sores in the mouth, throat, nose, genitals and eyes and may be preceded by fever and flu-like symptoms . Stop taking Eprex if you experience these symptoms and contact a doctor or hospital immediately. See also section 2.
Very common side effects are
These can occur in more than 1 in 10 people
- Diarrhea
- Nausea
- Vomiting
- Fever
- Narrow airways such as nasal congestion and sore throat have been reported in patients with kidney disease who are not yet undergoing dialysis .
Common side effects are
These can occur in up to 1 in 10 people
- High blood pressure . Headaches , especially sudden, stabbing migraine-like headaches, feelings of confusion or seizures can be signs of a sudden increase in blood pressure . This requires urgent treatment. High blood pressure may require medication (or adjustment of any medication you are already taking for high blood pressure ).
- Blood clots (including deep vein thrombosis and embolism) that may require urgent treatment. You may experience chest pain, shortness of breath, painful swelling and redness , usually in the leg , as symptoms.
- Cough
- Skin rash which may be caused by an allergic reaction .
- Skeletal or muscle pain
- Influenza-like symptoms , such as headache, joint pain and pain, feeling weak, chills, fatigue and dizziness. These may be more common at the beginning of treatment. If you get these symptoms by intravenous injection , they can be avoided in the future by giving injection more slowly.
- Redness , burning and pain at the injection site
- Swelling of the ankles, feet and fingers
- Pain in arms or legs
Uncommon side effects are
These can occur in up to 1 in 100 people
- High levels of potassium in the blood which can cause abnormal heart rhythms (this is a very common side effect in patients undergoing dialysis )
- Seizures
- Nasal congestion or narrow airways
- Allergic reaction
- Hives
Rare side effects are
These can occur in up to 1 in 1,000 people
- Symptoms of erythroblastopenia
Erythroblastopenia is the inability to produce enough red blood cells in the bone marrow . This can lead to sudden and severe anemia . Symptoms of this are:
- unusual fatigue
- dizziness
- shortness of breath .
Erythroblastopenia has been reported in very rare cases, mainly in patients with kidney disease, after several months to years of treatment with Eprex and other products that stimulate the formation of red blood cells .
- An increase in the level of small blood cells (so-called platelets ), which are normally involved in the formation of blood clots, can occur, especially at the beginning of treatment. Your doctor will check this.
- Severe allergic reaction which may include:
- swollen face, swollen lips, swollen mouth, tongue or throat
- difficulty swallowing or breathing
- itchy rash ( hives )
- Problems with the blood that can cause pain, dark colored urine or increased sensitivity of the skin to sunlight ( porphyria )
If you are undergoing hemodialysis:
- Blood clots ( thrombosis ) can form in your dialysis shunt. There is a greater risk of this if you have low blood pressure or if there are complications with your fistula .
- Blood clots can also form in your hemodialysis system. Your doctor may decide to increase your heparin dose during dialysis .
Tell your doctor or nurse immediately if you notice any of these side effects , or if you notice any other side effects while you are being treated with Eprex.
5. How to store Eprex
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton and label after EXP. The expiration date is the last day of the specified month.
Store in a refrigerator (2 ° C – 8 ° C). You can remove Eprex from the refrigerator and store at room temperature (maximum 25ºC) for a maximum of 3 days. As soon as the syringe has been removed from the refrigerator and has reached room temperature (maximum 25ºC), it must be used within 3 days or discarded.
Do not freeze or shake.
Store in the original package. Sensitive to light.
Do not use this medicine if you notice that the seal is broken, if the liquid has discolored or if you can see particles floating in it. Discard the medicine if any of these are observed.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the packaging and other information
Content declaration
- The active substance is epoetin alfa (for quantity see table below).
- The other ingredients are: polysorbate 80, sodium chloride, sodium dihydrogen phosphate dihydrate, disodium phosphate dihydrate, glycine and water for injections.
What the medicine looks like and the contents of the pack
Eprex is supplied as a solution for injection in pre-filled syringes. The pre-filled syringes are equipped with the PROTECS needle protection device (see table below). Eprex is a clear, colorless solution.
| Packaging type | Corresponding presentations in quantity / volume for each strength | Amount of epoetin alfa |
| Packs of 6 pre-filled syringes with PROTECS needle protection device | 2,000 IU / ml:1,000 IU / 0.5 ml | 8.4 micrograms |
| 4,000 IU / ml:2,000 IU / 0.5 ml | 16.8 micrograms | |
| 10,000 IU / ml: | ||
| 3,000 IU / 0.3 ml | 25.2 micrograms | |
| 4,000 IU / 0.4 ml | 33.6 micrograms | |
| 5,000 IU / 0.5 ml | 42.0 micrograms | |
| 6,000 IU / 0.6 ml | 50.4 micrograms | |
| 8,000 IU / 0.8 ml | 67.2 micrograms | |
| 10,000 IU / 1 ml | 84.0 micrograms | |
| Packs of 1 pre-filled syringe with PROTECS needle protection device | 20,000 IU / 0.5 ml | 168 micrograms |
| 30,000 IU / 0.75 ml | 252 micrograms | |
| 40,000 IU / 1 ml | 336 micrograms | |
| Packs of 4 pre-filled syringes with PROTECS needle protection device | 20,000 IU / 0.5 ml | 168 micrograms |
| 30,000 IU / 0.75 ml | 252 micrograms | |
| 40,000 IU / 1 ml | 336 micrograms | |
| Packs of 6 pre-filled syringes with PROTECS needle protection device | 20,000 IU / 0.5 ml | 168 micrograms |
| 30,000 IU / 0.75 ml | 252 micrograms | |
| 40,000 IU / 1 ml | 336 micrograms |
Not all pack sizes may be marketed.
Marketing Authorisation Holder
Janssen-Cilag AB, Box 4042, 169 04 Solna.
Manufacturer
Janssen Biologics BV, Einsteinweg 101, 2333 CB, Leiden, The Netherlands