2 mg, 8 mg sublingual resoriblets
buprenorphine
1. What Buprenorphine Actavis is and what it is used for
Buprenorphine Actavis is a medicine used to treat opioids (drugs).
Buprenorphine Actavis is used as part of a medical, social, and psychological treatment program for patients addicted to opioids (drugs). A sublingual travel tablet is a tablet that is placed under the tongue and allowed to dissolve.
The treatment is prescribed and monitored by doctors who specialize in the treatment of drug addiction.
Buprenorphine Actavis sublingual resoriblet is only for use in adults and adolescents over 15 years of age.
Buprenorphine contained in Buprenorphine Actavis may also be approved for the treatment of other conditions not mentioned in this product information. Ask your doctor, pharmacist or other healthcare professional if you have any further questions and always follow their instructions.
What you need to know before you use Buprenorphine Actavis
Do not use Buprenorphine Actavis
- if you are allergic to buprenorphine or any of the other ingredients of this medicine (listed in section 6).
- if you have severe breathing problems
- if you have severe hepatic impairment
- if you are addicted to alcohol or suffer from delirium tremens (“shaking” and hallucinations).
Warnings and cautions
Tell your doctor if you have any of the following diseases before treatment or develop them during treatment, as your doctor may need to reduce the dose of Buprenorphine Actavis or give you further treatment to control them:
- asthma or other respiratory problems
- liver or kidney problems. If you have a severe hepatic impairment, do not take buprenorphine.
- head injuries or brain disease
- low blood pressure
- enlarged prostate, which can make it difficult to shed water
- difficulty urinating due to narrowing of the urethra (urethral stenosis).
- depression or other conditions treated with antidepressants. If these medicines are used together with Buprenorphine Actavis, it may lead to the serotonergic syndrome, a condition that can be life-threatening (see “Other medicines and Buprenorphine Actavis”).
Misuse, abuse, and attempts to deceive
Misuse, especially by injecting high doses is dangerous and can be fatal.
Severe cases of infection, sometimes life-threatening, can occur with intravenous abuse of Buprenorphine Actavis.
Some people have died of respiratory depression (severe respiratory arrest) due to misuse of buprenorphine or ingestion in combination with other centrally acting substances such as alcohol, benzodiazepines (medicines used to treat anxiety or sleep disorders), or other opioids.
Cases of acute liver damage (liver problems) have been reported in connection with misuse, especially during intravenous administration and at high doses. These injuries may be due to specific circumstances, such as viral infections ( chronic hepatitis C), alcohol abuse, anorexia, or concomitant use of certain other medicines (for example antiretroviral nucleoside analogs, acetylsalicylic acid, amiodarone, isoniazid, and valproate ). If you have symptoms such as severe fatigue, no appetite, itching, or if your skin or eyes are yellow, contact your doctor immediately so that you can receive appropriate treatment.
This medicine may cause:
- withdrawal symptoms if you take it less than six hours after using drugs (morphine, heroin, or other products) or less than 24 hours after using methadone.
- drowsiness, which can be aggravated if you also drink alcohol or take sedatives or anxiolytics. If you become drowsy, do not drive or use machines.
- the sudden drop in blood pressure, which makes you feel dizzy if you get up too fast from a sitting or lying position
- drug addiction
Buprenorphine Actavis may mask pain, which is a sign of another disease. Do not forget to tell your doctor that you are taking this medicine.
The risk of serious side effects is greater if you use opiates, alcohol, sedatives, and hypnotics, especially benzodiazepines.
Discontinuation of treatment may lead to withdrawal symptoms.
Children and young people
Buprenorphine Actavis should not be used in children and adolescents under 15 years of age due to a lack of data on safety and efficacy.
Other medicines and Buprenorphine Actavis
Tell your doctor or pharmacist if you are taking, have recently taken or might take any other medicines.
Before you take Buprenorphine Actavis, you must tell your doctor if you are taking any of the following medicines:
- antidepressants such as moclobemide, tranylcypromine, citalopram , escitalopram, fluoxetine , fluvoxamine, paroxetine , sertraline , duloxetine, venlafaxine , amitriptyline, doxepine or trimipramine. These medicines can affect or be affected by Buprenorphine Actavis and you may experience symptoms such as involuntary rhythmic muscle twitching, even in the muscles that control eye movements, agitation, hallucinations, coma, heavy sweating, tremors, intensified reflexes, tense muscles, and body temperature above 38 ° C . Contact your doctor if you experience such symptoms.
- other drugs with sedatives, including sleep-inducing antihistamines, certain antidepressants, and clonidine (a treatment for high blood pressure, migraines, menopausal symptoms).
- potent painkillers ( opioids ) and cough medicines containing opiate-related substances such as methadone, dextropropoxyphene, codeine, dextromethorphan, and noscapine.
- barbiturates and other medicines used to treat sleep disorders, anxiety, or antispasmodics.
- monoamine oxidase inhibitors (a type of antidepressant).
- antipsychotic drugs.
- gestodene (a contraceptive pill ).
- drugs for the treatment of HIV / AIDS ( protease inhibitors ) including indinavir, ritonavir, nelfinavir, and saquinavir.
- antiepileptic (antispasmodic) drugs including phenobarbital, carbamazepine, and phenytoin.
- antibiotics including rifampicin, erythromycin, troleandomycin.
- medicines for fungal infections, including ketoconazole and itraconazole.
Concomitant use of Buprenorphine Actavis and sedatives or medicines for insomnia such as benzodiazepines or similar medicines increases the risk of drowsiness, difficulty breathing ( respiratory depression ), coma and may be life-threatening. Due to this, concomitant use should only be considered when other treatment options are not possible. If your doctor prescribes Buprenorphine Actavis at the same time as sedatives or medicines for insomnia, the dose and treatment time should be limited by your doctor.
Tell your doctor if you are taking any sedatives or medicines for sleep disorders and carefully follow your doctor’s dose recommendations. It may be helpful to inform friends or relatives about paying attention to the signs and symptoms described above. Contact a doctor if you experience any of these symptoms.
Buprenorphine Actavis with alcohol
Do not drink alcohol while being treated with buprenorphine. Alcohol increases the sedative effect of buprenorphine, which can make driving and using machines dangerous.
Pregnancy and breastfeeding
If you are pregnant or breast-feeding, think you may be pregnant or are planning to have a baby, ask your doctor or pharmacist for advice before taking this medicine.
Pregnancy
Tell your doctor if you are pregnant or trying to become pregnant before using Buprenorphine Actavis. Tell your doctor immediately if you become pregnant during treatment with Buprenorphine Actavis. Buprenorphine Actavis should only be used during pregnancy if the benefits outweigh the potential risks.
Neonatal withdrawal symptoms including respiratory depression have been reported following the treatment of mothers in the latter part of pregnancy. Your doctor will decide if you should be treated with Buprenorphine Actavis.
Breast-feeding
As this medicine passes into breast milk and may adversely affect the breast-fed baby, you should stop breast-feeding while using Buprenorphine Actavis.
Driving and using machines
Buprenorphine may cause drowsiness. If you feel tired, do not drive or use machines.
You are responsible for assessing whether you are fit to drive a motor vehicle or perform work that requires increased attention. One of the factors that can affect your ability in these respects is the use of drugs due to their effects and / or side effects . Descriptions of these effects and side effects can be found in other sections. Read all the information in this leaflet for guidance. If you are not sure, talk to your doctor or pharmacist.
Buprenorphine Actavis contains lactose, para-orange, and sodium
Buprenorphine Actavis contains lactose
If you have been told by your doctor that you have an intolerance to some sugars, contact your doctor before taking this medicine.
Buprenorphine Actavis contains para-orange
Buprenorphine Actavis 2 mg and 8 mg sublingual tablet tablets also contain the dye para-orange (E110), which may cause allergic reactions.
Buprenorphine Actavis contains sodium
This medicine contains less than 1 mmol (23 mg) sodium per travel tablet, ie essentially ‘sodium-free’.
How to use Buprenorphine Actavis
Method of administration
Always use this medicine exactly as your doctor has told you. Ask your doctor or pharmacist if you are unsure.
Buprenorphine Actavis resoriblets are used sublingually. This means that you should place the travel tablet under the tongue and let it dissolve. This is the only way to take resoriblet. They should NOT be chewed, crushed, or swallowed whole, because then they will not work properly, and you may have withdrawal symptoms.
Take a dose once a day, unless otherwise directed by your doctor.
Your doctor will determine the optimal dose for you. During your treatment, your doctor may adjust the dose depending on your response. To get the most benefit from Buprenorphine Actavis, you must tell your doctor about all the medicines you are taking including alcohol, medicines containing alcohol and drugs, and any prescription medicines that you are taking that have not been prescribed for you by your doctor.
After the first dose of Buprenorphine Actavis, it is possible that you may have some opiate withdrawal symptoms, see section 4 “Possible side effects”.
Impaired kidney or liver function
Your dose may need to be reduced if you have kidney or liver problems. Talk to your doctor. If you suffer from a severe hepatic impairment, do not use Buprenorphine Actavis.
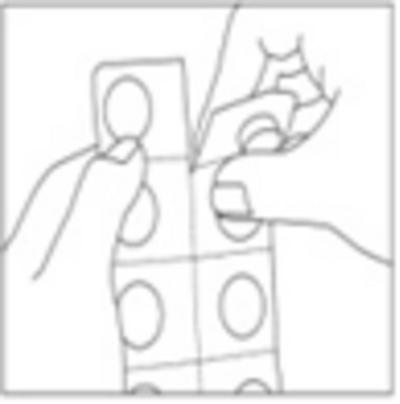
[Only for child-resistant blister packs:]
Instructions for use of child-resistant blisters:
- Do not push the tablet out of your pocket directly
- Separate a blister cell from the strip at the perforation
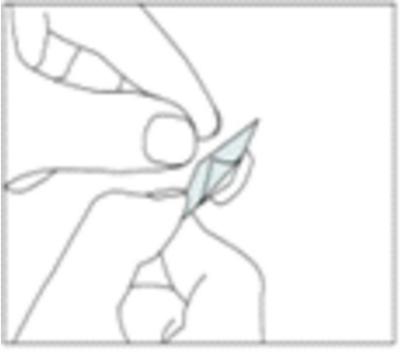
Carefully pull off the back of the arrow
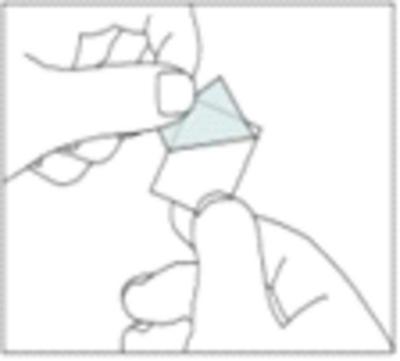
4. Push the tablet through the foil
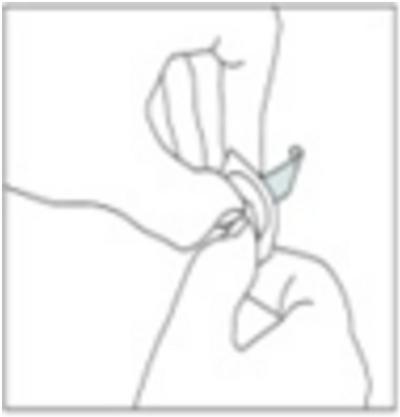
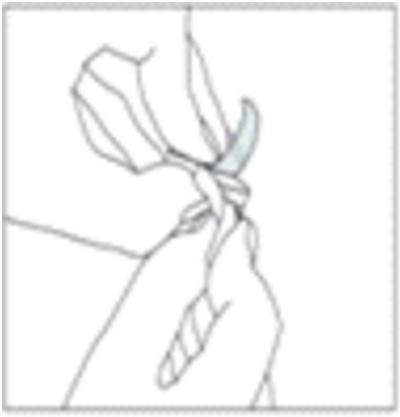
5. Place the tablet under your tongue
Treatment time
The duration of treatment will be determined individually by your doctor.
After a period of successful treatment, your doctor may gradually reduce the dose to a lower maintenance dose. Depending on your condition, the dose of Buprenorphine Actavis may continue to be reduced under close medical supervision until it can be discontinued completely.
Do not change the treatment in any way or end the treatment without the consent of the doctor treating you.
The effect of this treatment depends on the dose in combination with simultaneous medical, psychological, and social treatment.
If you have the impression that the effect of Buprenorphine Actavis is too strong or insufficient, talk to your doctor or pharmacist.
Symptoms of an overdose may include difficulty breathing, slow breathing, or heart problems. Poisoning has been observed after incorrect use (overdose or misuse) and in the worst case, it can result in respiratory arrest, heart failure, and/or liver damage.
If you forget to use Buprenorphine Actavis
Contact your doctor if you have forgotten to take Buprenorphine Actavis. Do not take a double dose to make up for a forgotten dose, unless your doctor instructs you to do so.
If you stop using Buprenorphine Actavis
Do not stop using Buprenorphine Actavis suddenly, as this may lead to withdrawal symptoms (sweating, anxiety, and restlessness). Do not stop the treatment on your own, but ask your doctor how to end the treatment.
If you have any further questions on the use of this product, ask your doctor or pharmacist.
4. Possible side effects
Like all medicines, this medicine can cause side effects, although not everybody gets them.
Contact your doctor immediately or seek emergency care if you experience very rare (may affect up to 1 in 10,000 users) allergic reactions, such as:
- difficulty breathing, wheezing and swelling of the eyes, lips, throat, tongue, or hands.
- skin rash, hives, and itching .
Also contact your doctor immediately if you experience less common side effects(may affect up to 1 in 100 people), such as:
- severe fatigue, loss of appetite, or yellowing of the skin and eyes. This can be a symptom of liver damage such as cell death in the liver (liver necrosis).
Improper use of this medicine by injecting it may cause withdrawal symptoms, infections, other skin reactions, and any serious liver problems – see “Warnings and Precautions”.
After the first dose of Buprenorphine Actavis, you may experience some of the symptoms of opioid withdrawal, see section 3 “How to use Buprenorphine Actavis”.
Very common (may affect more than 1 user in 10)
- insomnia
- feeling weak
- withdrawal symptoms
Common (may affect up to 1 in 10 people)
- headache
- fainting
- dizziness
- anxiety
- nervousness
- constipation
- nausea
- vomiting
- diarrhea
- abdominal pain
- tear flow disorder
- snuva
- drowsiness
- drop in blood pressure when changing from sitting or lying position to standing position
- sweating
- back pain
- overindulge
- abnormal ECG
Uncommon (may affect up to 1 in 100 people)
- hallucinations
- severe difficulty breathing ( respiratory depression )
Reporting of side effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet. You can also report side effects directly to the Medical Products Agency, www.lakemedelsverket.se. By reporting side effects, you can help increase drug safety information.
5. How to store Buprenorphine Actavis
Keep this medicine out of the sight and reach of children.
Do not use this medicine after the expiry date which is stated on the carton, jar, and blister after “EXP”. The expiration date is the last day of the specified month.
Blister packs: Do not store above 25 ° C. Store in the original package. Moisture sensitive.
Jars: Do not store above 30 ° C. Close the jar tightly. Moisture sensitive.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. These measures will help to protect the environment.
6. Contents of the packaging and other information
Content declaration
- The active substance is buprenorphine (as buprenorphine hydrochloride). Each travel tablet contains 2 mg or 8 mg of buprenorphine.
- The other ingredients are magnesium stearate, sodium citrate, povidone, citric acid, pregelatinized maize starch, lactose monohydrate , crospovidone, mannitol, and para-orange (E110).
What the medicine looks like and the contents of the pack
Buprenorphine Actavis 2 mg sublingual resoriblets are non-film coated, light orange, 5×8 mm oval, and biconvex with “B” on one side.
Buprenorphine Actavis 8 mg sublingual resoriblets are non-film coated, light orange, 7.35×13.35 mm oval, and biconvex with “B” on one side.
Packaging: Buprenorphine Actavis is available in cans, standard blister packs, and child-resistant blister packs of 1, 7, 20, 24, 28, 48 and 50 resoriblets.
Not all pack sizes may be marketed.
Marketing Authorization Holder and Manufacturer
Marketing Authorisation Holder
Actavis Group PTC ehf.
Reykjavikurvegur 76-78
IS-220 Hafnarfjordur
Iceland
Manufacturer
Actavis Group PTC ehf.
Reykjavikurvegur 76-78
IS-220 Hafnarfjordur
Iceland
Accord UK Limited
Whiddon Valley
Barnstaple
EX32 8NS
UK
Tjoapack Netherlands BV
New Donk 9
4879 AC Etten-Leur
Netherlands